2497
Extensive cortical involvement in patients with Huntington’s Disease as measured from diffusion MRI1Medical Imaging and Radiological Sciences, Chang-Gung University, Taoyuan City, Taiwan, 2Neurology, Chang Gung Memorial Hospital, Linkou, Taoyuan City, Taiwan, 3Neuroscience Research Center, Chang Gung Memorial Hospital, Linkou, Taoyuan City, Taiwan
Synopsis
Huntington’s Disease (HD) is a neurodegenerative disease would result in atrophy in basal ganglia especially in caudate nucleus and putamen in the early stage of the disease. The cortical parcellation algorithm was applied to evaluate the cortical involvement in the patients with HD by using diffusion MRI and compared with voxel based morphometry. The mean diffusivity is feasible in the brain of patients with HD, which is more sensitive than the morphometric changes. Therefore mean diffusivity could be a potential image based biomarker for monitoring HD progression.
INTRODUCTION
Huntington’s Disease (HD) is a neurodegenerative disease caused by a mutated gene associated with the abnormal aggregation of huntingtin protein, which results in the neuron death1. MRI reported atrophy in basal ganglia especially in caudate nucleus and putamen in the early stage of the disease2. Because diffusion tensor imaging (DTI) can be more sensitive to the underlying microstructural changes than volumetric assessment, it provides great potential for sensitive detection before the symptom onset. Therefore, the current study aimed to evaluate the cortical involvement in the patients with HD by using diffusion MRI and compared with voxel based morphometry.METHODS
The study was approved by the Institutional Review Board, and complied with the Declaration of Helsinki. All subjects gave written informed consent before participating in the study. Eleven patients with HD (aged 53.1±12.9 years old) and thirteen age range-matched normal controls (NC, aged 45.8±10.3 years old) were recruited in the study. Images were acquired from 3T MR scanner (Trio, Magnetom, Siemens, Erlangen Germany), including T1-weighted magnetisation-prepared rapid (MPRAGE) acquisition gradient echo (TR/TE = 1700ms/2.63ms, flip angle = 9°, voxel size=1*1*1 mm) and diffusion tensor imaging (EPI sequence, TR/TE = 5200ms/92ms, voxel size=2*2*3 mm, 12 directions, b-value=1000 sec/mm2). The image processing procedure was followed by Lu et al3. Mean Diffusivity (MD) were calculated from the diffusion tensor, which was subsequently normalized and parcellated into 116 regions of interest (ROI) based on the Automatic Anatomical Labeling (AAL) template. Mann-Whitney U test was performed to identify the regions with significant difference in the parcellated cortical MD value. Bonferroni correction was applied to correct for multiple comparison (p-value<0.00043, Bonferroni: 0.05/116). The correlation between MD and Unified Huntington’s Disease Rating Scale (UHDRS) was examined by using Spearman’s rho (p<0.05). Standard voxel-based morphometry (VBM) was used to investigate the voxel-wise structural changes between two groups (FDR p<0.01).RESULTS
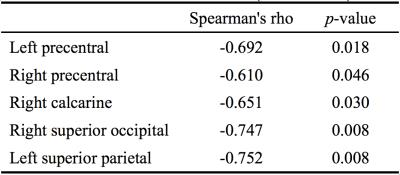
Figure 1 showed the regions with gray matter atrophy and overlaid with increased MD. Significant increase in MD can be noticed in 62 out of 116 AAL regions. Compared with normal, significant difference can be noticed in basal ganglia, including right caudate nucleus (61.3%), left putamen (20.3%), right putamen (18.4%) and left thalamus (13.4%). The atrophic regions from VBM analysis were mainly located in bilateral caudate nucleus and posterior lobe in cerebellum. Additional regions were noticed in left thalamus, parahippocampal gyrus, amygdala, and right lingual sporadically. Significant correlation between MD and UHDRS (motor domain) excan be noticed in 6 regions, including left precentral (spearman’s rho=-0.692, p=0.018) and superior parietal (spearman’s rho=-0.752, p=0.008), right precentral (spearman’s rho=-0.610, p=0.046), calcarine (spearman’s rho=-0.651, p=0.030), superior occipital (spearman’s rho=-0.747, p=0.008), and right superior parietal (spearman’s rho=-0.620, p=0.042) lobes (Table 1).DISCUSSION
HD patients typically showed significant chorea. Therefore, prolonged image acquisition, especially diffusion MRI, was often regarded as non-useable if not impossible. The current study demonstrated the feasibility of the acquisition of diffusion tensor imaging and high resolution T1-weighted MPRAGE in HD patients. The atrophy region is largely in line with the regions of significantly increased MD, which is consistent with the observation of increased MD as attributed to an increased extracellular space as a result of potential cell death. The locations of increased MD can be related to the motor abnormality in the patients, where the previous study reported significant atrophy4. However, the affected regions extended further outside basal ganglia, which might suggest increased sensitivity in MD than the simple morphometric measurement. The correlation analysis indicated the involvement in regions responsible for visual or cognitive functions5. Both the changes and correlation with MD from extensive cortical areas might suggest that the disease is more than a movement disorder. Further investigation might be necessary to bring new insight to the underlying mechanism and to improve the treatment planning of the disease.CONCLUSION
Mean diffusivity is feasible in the brain of patients with HD, which is more sensitive than the morphometric changes. Therefore mean diffusivity could be a potential image based biomarker for monitoring Huntington’s disease.Acknowledgements
No acknowledgement found.References
1. Arrasate M, Mitra S, et al. Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature. 2004;431:805-810.
2. Harris GJ, Pearlson GD, Peyser CE, et al. Putamen volume reduction on magnetic resonance imaging exceeds caudate changes in mild Huntington’s disease. Annals of Neurology. 1992;31:69-75.
3. Lu CS, Ng SH, Weng YH, et al. Alterations of diffusion tensor MRI parameters in the brains of patients with Parkinson’s disease compared with normal brains: possible diagnostic use. European radiology. 2016 ;26:3978-3988.
4. Halliday GM et al. Regional specificity of brain atrophy in Huntington's disease. Experimental Neurology. 1998: 663-672.
5. Jessica A. Grahn et al. The cognitive functions of the caudate nucleus. Progress in Neurobiology. 2008: 141–155.
Figures