2492
Longitudinal Volume Change of Hippocampal Subfields and Cognitive Decline in Parkinson's Disease1Department of Radiology, Department of Radiology, Southwest Hospital, Third Military Medical University, Chongqing, People's Republic of China, 2Collaboration NEA, Siemens Healthcare Ltd., Shanghai, P.R. China, People's Republic of China, 3Collaboration NEA, Siemens Healthcare Ltd., Beijing, P.R. China, People's Republic of China
Synopsis
We try to find out the longitudinal volume change of different hippocampal subfields in patients with PD with and without cognitive decline using magnetic resonance image (MRI). Our result shows that there is cross-sectional and longitudinal regional atrophy of specific hippocampal subfields in PD, which becomes more severe and is further extended to the bilateral CA2-3 and CA4-DG subfields in patients with cognitive decline. These results corroborate neuropathological findings and add novel information about the involvement of the hippocampus in the cognitive dysfunction of PD.
Background
Neuropathological studies have shown that the hippocampus is affected in Parkinson’s disease (PD) with cognitive impairment 1,2. Our goal was to assess the longitudinal volume change of different hippocampal subfields in patients with PD with and without cognitive decline using magnetic resonance image (MRI) and test their association with global cognitive decline.Methods
A total of 28 nondemented PD patients and 27 neurologically unimpaired elderly controls matched by age and gender were enrolled in this study. All PD patients that were followed up after 2 years were divided into two groups: PD without cognitive decline (n=15) and PD with cognitive decline (n=13). Global cognitive status was assessed with the Mini Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA). The MRI data were acquired using a MAGNETOM Trio 3T MR scanner (Siemens). For each subject, three-dimensional T1-weighted anatomical images were acquired in a sagittal orientation using the following volumetric three-dimensional magnetization-prepared rapid gradient–echo (MP-RAGE) sequence (TR = 1900 ms, TE = 2.52 ms, flip angle = 9°, slice thickness = 1 mm, 176 slices, FOV = 256 mm × 256 mm, matrix size = 256 × 256 and voxel size = 1 mm × 1 mm × 1 mm). In the follow-up group, patients were rescanned after two years (mean 899 days, SD 36) with the same sequence in the same MR scanner. The volumes of seven hippocampal subfields were assessed, including the cornu armonis (CA) sectors, subiculum, presubiculum, hippocampal tail, hippocampal fissure, fimbria, and the dentate gyrus (DG). Volumetric segmentation was performed with the Freesurfer image analysis suite (http://surfer.nmr.mgh.harvard.edu/)3-5. Hippocampal subfield volumes between PD group and healthy control at baseline were compared using the analysis of covariance (ANCOVA) and under the control of age, sex, education, and intracranial volume (ICV). Longitudinal changes on hippocampal subfield volume were also determined using repeated measures ANCOVA (mixed-effects model) and making the group as a between-subjects factor (PD without cognitive decline group vs. PD with cognitive decline group) and the time (baseline, follow-up) as a within-subjects repeated measure and the age as a covariate 6,7. The correlation analyses between MMSE or MoCA scores and the total or subfield hippocampal volumes were further investigated within each group.Results
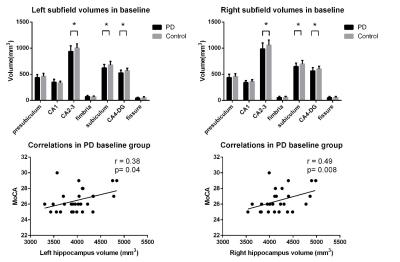
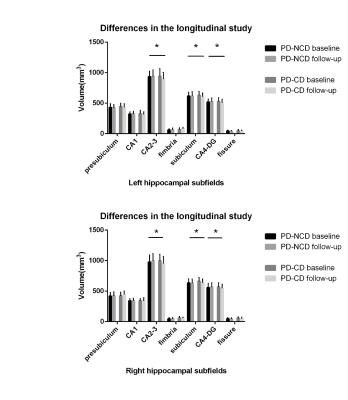
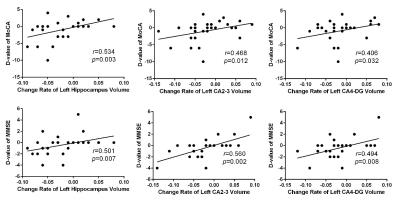
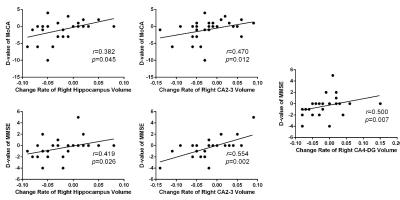
In the cross sectional study, bilateral hippocampal volume was smaller in PD patients compared to healthy controls (left: P = 0.009, right: P = 0.009). Meanwhile, the bilateral CA2-3 (left: P = 0.014, right: P = 0.01), CA4-DG (dentate gyrus) (left: P = 0.002, right: P = 0.023), subiculum (left: P = 0.003, right: P = 0.008) subfields were all significantly decreased in the PD patients (Figure 1). Significant correlations were found between MoCA score and total hippocampus volume in PD patients (left: r=0.38, P = 0.04; right: r=0.49, P = 0.008) (Figure 1). In the follow-up group, bilateral CA2-3 (left: P = 0.006, right: P = 0.004), CA4-DG (left: P = 0.004, right: P = 0.004) and subiculum (left: P = 0.027, right: P = 0.046) subfields of PD patients with cognitive decline were much smaller than those of PD patients without cognitive decline (Figure 2). Significant correlations were found between longitudinal change of the MMSE or MoCA scores and percent change rate of the total bilateral hippocampal, or bilateral CA 2-3, or CA4-DG in all PD patients (Figure 3,4).Conclusions
These data show there is cross-sectional and longitudinal regional atrophy of specific hippocampal subfields in PD, which becomes more severe and is further extended to the bilateral CA2-3 and CA4-DG subfields in patients with cognitive decline. Our findings indicate that global cognition deficits are associated with volume loss in subfields that act as input regions in the hippocampal circuit, suggesting the degeneration in these regions could be responsible for cognitive dysfunction in PD.Acknowledgements
No acknowledgement found.References
1.Junque C, Ramirez-Ruiz B, Tolosa E, et al. Amygdalar and hippocampal MRI volumetric reductions in Parkinson's disease with dementia. Movement disorders : official journal of the Movement Disorder Society 2005;20(5):540-544.
2.Carlesimo GA, Piras F, Assogna F, Pontieri FE, Caltagirone C, Spalletta G. Hippocampal abnormalities and memory deficits in Parkinson disease: a multimodal imaging study. Neurology 2012;78(24):1939-1945.
3.Van Leemput K, Bakkour A, Benner T, et al. Automated segmentation of hippocampal subfields from ultra-high resolution in vivo MRI. Hippocampus 2009;19(6):549-557.
4.Kandiah N, Zhang A, Cenina AR, Au WL, Nadkarni N, Tan LC. Montreal Cognitive Assessment for the screening and prediction of cognitive decline in early Parkinson's disease. Parkinsonism & related disorders 2014;20(11):1145-1148.
5.Khan W, Westman E, Jones N, et al. Automated hippocampal subfield measures as predictors of conversion from mild cognitive impairment to Alzheimer’s disease in two independent cohorts. Brain topography 2015;28(5):746-759
6.Du AT, Schuff N, Amend D, et al. Magnetic resonance imaging of the entorhinal cortex and hippocampus in mild cognitive impairment and Alzheimer's disease. Journal of Neurology, Neurosurgery & Psychiatry 2001;71(4):441-447.
7.Ibarretxe-Bilbao N, Junque C, Segura B, et al. Progression of cortical thinning in early Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society 2012;27(14):1746-1753.
Figures