2487
Predominance of Odor-related Functional Decline in the Primary Olfactory Cortex of Early-stage Parkinson’s Disease1Radiology, Penn State College of Medicine, Hershey, PA, United States, 2Neurology, Penn State College of Medicine, Hershey, PA, United States, 3Neural & Behavioral Sciences, Penn State College of Medicine, Hershey, PA, United States, 4Neurosurgery, Penn State College of Medicine, Hershey, PA, United States
Synopsis
The primary olfactory cortex (POC) responds to both odor-smelling and sniffing. It is not known if there are deficits in the sniffing-related or odor-related functional activities in the POC of early-stage Parkinson’s disease (PD). Here we report significant PD-related deficit in the odor-related POC activation, while the sniffing-related activation was not significantly affected. These results suggest that olfactory deficits in early-stage PD are mainly due to the breakdown of the bottom-up mechanism. In addition, our finding of a negative correlation between the UPDRS-3 score and the odor-related POC activation suggests a surrogate marker for the clinical severity in early-stage PD.
INTRODUCTION
Hyposmia has been reported to occur in the majority of early-stage Parkinson’s disease (PD)1-3. The sniffing function has also been reported to be affected in PD4. Despite considerable progress in understanding the pathophysiology of the disease, the mechanism causing hyposmia in PD is still unclear. The primary olfactory cortex (POC) responds to both odor-smelling and sniffing. Currently, it is not known if there are deficits in the sniffing-related or odor-related functional activities in the POC of PD. Given that there is early PD-related neurodegeneration in the olfactory bulb and anterior olfactory nucleus3, 5, we hypothesized that there are PD-related functional deficits in the POC at the early stage of disease. To test this hypothesis, we studied the functional changes of the POC related to the odor-smelling and sniffing functions in early-stage PD subjects. Furthermore, we sought to determine the relevance between sniffing and odor-related activities in the POC and the clinical status of the disease.METHODS
Human Subjects Twenty-seven H&Y stage I-II early onset idiopathic PD subjects (mean 56.0 ± 4.3 years; 9 females, disease duration 3.5 ± 2.3 years) participate in the study. Their motor function deficits were evaluated with the Part 3 of MDS-Unified Parkinson’s Disease Rating Scale (UPDRS-3). For comparison, 22 age/sex-matched healthy subjects (54.2 ± 6.2 years, 13 females) participated as negative controls (HC). There was no significant difference in the age or sex distributions between the two groups.
fMRI Examination and Data Analysis Psychophysical testing was performed on all the subjects for their smell identification function using the University of Pennsylvania Smell Identification Test (UPSIT) and smell detection threshold using the OLFACT-C Olfactory Threshold Test (Osmic Enterprises, Inc.). fMRI examination was conducted on a Siemens 3 T scanner with an 8-channel head coil, a BOLD-sensitive EPI sequence with TR 2s, TE 30 ms, FA 90°, and 2.8 mm×2.8 mm image resolution, and an odor-sniffing stimulation paradigm6. The respiration data were processed with ONSET7. The fMRI data were processed with SPM8. Statistical parametric maps for odor-sniffing, odorless-sniffing, and odor-related activation were generated at both the individual and group level. The comparison between the olfactory activations in the HC and PD subjects was conducted using ANOVA with age as a confounding factor. The correlations between the BOLD signal in the POC of PD subjects with the clinical status were evaluated using multiple regressions with age as a confounding factor.
RESULTS
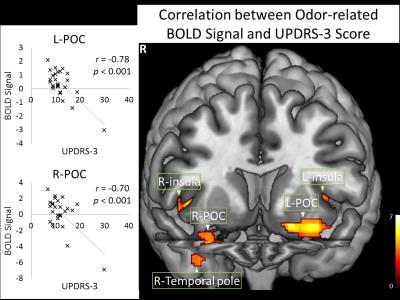
Psychophysical tests showed significant olfactory functional deficits in the PD subjects with UPSIT score of 21.7 ± 9.5 and smell threshold of 6.6 ± 3.2, both were significantly lower than the HCs (UPSIT score 34.9 ± 2.7 and smell threshold 9.8 ± 2.4) (two sample t-tests, p < 0.001, respectively). Both odor-sniffing and odorless-sniffing induced significant activation in the HC and PD subjects in all the central olfactory structures. There was no significant difference between the HC and PD groups in either odor-sniffing or odorless-sniffing-induced activations. Odor-related activation was observed in the HCs at the bilateral POC, left hippocampus, and right insular cortex. In contrast, odor-related activation was observed only at the left OFC of patients, but not in the POCs. There were significant negative correlations between the patients’ UPDRS-3 score and odor-related BOLD signals in the bilateral POC (Figure 1). There was no significant correlation between the disease duration and the BOLD signals in the central olfactory structures.DISCUSSION
In early-stage PD, there are significant deficits in the odor-related POC activation, while the sniffing-induced activation was not significantly affected. The demonstration of the predominance of odor-related functional decline in the POC of early-stage PD is important for understanding the mechanisms underlying olfactory deficits in this disease. From peripheral to central olfactory system the odor signal is transferred through a bottom-up mechanism. In contrast, the sniffing function in the central olfactory system presumably follows a top-down mechanism since it also involves attention and motor components. The functional activity in the POC responding to the odor-sniffing stimulation should be determined by the convolution of these two mechanisms. The predominance of odor-related functional decline in the POC of early-stage PD suggests that the cause of olfactory deficits is mainly due to the breakdown of the bottom-up mechanism, which is likely caused by the PD pathology in the olfactory bulb and olfactory track. The negative correlation between the UPDRS-3 score and odor-related BOLD signal in the POCs suggests the decrease of odor-related POC activity as a surrogate marker for the clinical severity of PD in its early stages.Acknowledgements
This study was supported by the DANA Foundation, and the Department of Radiology, Pennsylvania State University College of Medicine.References
1. Haehner A, Boesveldt S, Berendse HW, Mackay-Sim A, Fleischmann J, Silburn PA, Johnston AN, Mellick GD, Herting B, Reichmann H, Hummel T: Prevalence of smell loss in Parkinson's disease--a multicenter study, Parkinsonism Relat Disord 2009, 15:490-494.
2. Muller A, Mungersdorf M, Reichmann H, Strehle G, Hummel T: Olfactory function in Parkinsonian syndromes, J Clin Neurosci 2002, 9:521-524.
3. Doty RL, Deems DA, Stellar S: Olfactory dysfunction in parkinsonism: a general deficit unrelated to neurologic signs, disease stage, or disease duration, Neurology 1988, 38:1237-1244.
4. Sobel N, Thomason ME, Stappen I, Tanner CM, Tetrud JW, Bower JM, Sullivan EV, Gabrieli JD: An impairment in sniffing contributes to the olfactory impairment in Parkinson's disease, Proc Natl Acad Sci U S A 2001, 98:4154-4159.
5. Pearce RK, Hawkes CH, Daniel SE: The anterior olfactory nucleus in Parkinson's disease, Mov Disord 1995, 10:283-287
6. Wang J, Sun X, Yang QX: Early Aging Effect on the Function of the Human Central Olfactory System, J Gerontol A Biol Sci Med Sci 2016, doi: 10.1093/gerona/glw104.
7. Wang J, Sun X, Yang QX: Methods for olfactory fMRI studies: Implication of respiration, Hum Brain Mapp 2014, 35:3616-3624