2482
Robust method for detection of small variations in relaxation parameters and free water content in substantia nigra of Parkinson's disease patients.1Medical Imaging Physics, Institute of Neuroscience and Medicine, Forschungszentrum Jülich, Jülich, Germany, 2Department of Neurology, Faculty of Medicine, RWTH Aachen, JARA, Aachen, Germany, 3Nuclear Medicine and PET Center, Aarhus University, Aarhus, Denmark
Synopsis
Parkinson's disease patients were investigated in order to reveal changes inside region-of-interest – substantia nigra. 31 volunteers were scanned using a well-established, quantitative free water mapping protocol. The region-of-interest is too small to obtain reliable segmentation for region-based analysis. Therefore, statistical, voxel-wise analysis of registered quantitative maps was performed. It revealed a decrease in the metrics (free water content, T1, T2* and combination of all three) in the vicinity of substantia nigra. We conclude that the reduction in total free water content could be due to a disruption of the deep grey matter integrity.
Purpose
Parkinson's disease (PD) is a progressive neurodegenerative disorder.1 Its motor symptoms are linked to loss of dopaminergic neurons in the substantia nigra (SN).2 The underlying mechanisms (protein misfolding and aggregation, oxidative stress and mitochondrial dysfunction) are known and have been studied by multiple authors.3,4 The establishment of quantitative biomarkers is necessary for monitoring the disease. Recently, diffusion-based magnetic resonance imaging has been employed to observe changes in the fractional volume of free water in large cohorts of patients.5 Segmentation of the SN is extremely difficult, especially in patient cohorts, due to low contrast and insufficient resolution of MR images. Typically, gradient echo scans for water mapping have 1 mm in-plane resolution with a slice thickness of 2 mm and the SN therefore consists of approximately 300 voxels. However, the current study presents a different approach, where statistical analysis of quantitative, free water content-based metrics revealed the pathological region of the SN without manual segmentation.Methods
31 volunteers (18 patients and 13 controls) were scanned with a gradient echo based protocol optimized for water mapping and quantitative free water content as well as T1 and T2* maps (with a two-point method6) were obtained. Quantitative maps were registered to a common template (MNI) by means of the combination of linear (affine) and non-linear registration (demons) methods. After registration, metrics was defined as either T1, T2* or water content value or combination of all three. Then, two volumes were created; first, where each voxel was characterized by 18 values (for each metrics) from co-registered patients, and second, where each voxel was characterized by 13 values from co-registered controls. Finally, a voxel-wise comparison was performed by a simple two-means t-test with a null hypothesis stating, "there is no change in metrics" or "metrics increased" in order to detect changes in the vicinity of substantia nigra.Results
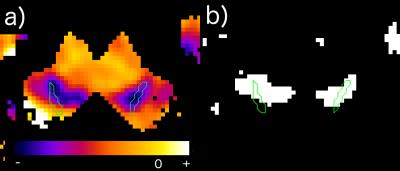
In each case, a decrease in metrics was observed which was, however, a combination of free water content acquired by two-point method and relaxation parameters (T1, T2*), which reveals the regions affected by the disease in the most prominent way. It has been shown that even in presence of only small variations of measured values (T1, T2*,free water content) between control group and patients, the null-hypothesis stating that "voxel has not been affected" can be rejected for consistent clusters inside and in the close vicinity of substantia nigra (see Fig.1). The size of these (instead of mean value for the region which is easily disturbed by partial volume effects) might become a good marker for monitoring disease progression. This also presents a concept of a "reverse-engineering" approach in (pathological) substantia nigra segmentation where instead of manually drawing the regions to extract it, one can simply join the affected voxels in order to obtain the valid contour.Discussion
Shortening of T1 and T2* times in the substantia nigra is in agreement with the literature7,8 and represents neuronal loss and iron accumulation in this structure. To our knowledge, reduction in free water content in substantia nigra is a new finding. Other studies have shown increase in fractional free water using diffusion MRI5 which is only the extracellular part of the water content. The methods used in this study indicate a shift as result of SN neurodegeneration. We argue that the reduction in total free water content could be due to disruption of the deep grey matter integrity and an increase in extracellular water might not be enough to compensate for the decrease in water content caused by neuronal cell death.Conclusion
This work presents a method to detect small changes in free water content and relaxation parameters in SN induced by the Parkinson’s disease, which are normally difficult to observe due to limited resolution and poor statistics "per subject" (few voxels of interest). Our method might provide a better understanding of the mechanisms controlling progression of Parkinson's disease. Finally, the presented approach can be abstracted to a more general method – that of "if-instead-of-how-much" and easily extrapolated to other pathologies affecting different brain regions even with completely different metrics used as imaging marker.Acknowledgements
No acknowledgement found.References
[1] R. E. Burke et al., "Axon degeneration in Parkinson's Disease," Experimental Neurology, vol. 246, pp 72-83, August 2013.
[2] A. Galvan et al., "Pathophysiology of Parkinsonism" Clinical Neurophysiology, vol 119(7), pp. 1459-74, Jul. 2008.
[3] V. Dias et al., "The role of Oxidative Stress in Parkinson's Disease", Journal of Parkinson's Disease, vol. 3(4), pp. 461-491, 2013.
[4] W. Dauer et al., "Parkinson's Disease: Mechanisms and Models", Neuron, vol. 39(6), pp. 889-909, September 2003.
[5] E. Ofori et al., “Increased free water in the substantia nigra of Parkinson’s disease: a single-site and multi-site study,” Neurobiology of Aging, vol. 36, no. 2, pp. 1097–1104, Feb. 2015.
[6] V. Gras, Z. Abbas, and N. J. Shah, “Spoiled FLASH MRI with Slice Selective Excitation?: Signal Equation with a Correction Term,” Concepts in Magnetic Resonance, vol. 42, no. April, pp. 89–100, 2013.
[7] S. Baudrexel et al., 'Quantitative mapping of T1 and T2* discloses nigral and brainstem pathology in early Parkinson's disease", Neuroimage, vol 51(2), pp. 512-20, 2010.
[8] P. Tofts, "Quantitative MRI of the Brain", 2003.
Figures