2478
Effects of myelin changes in Parkinson's Disease on motor performance1Department of Biomedical Engineering, University of British Columbia, Vancouver, BC, Canada, 2Pacific Pakinson's Research Centre, UBC Hospital, Vancouver, BC, Canada, 3Graduate Program in Neuroscience, University of British Columbia, 4Faculty of Medicine, Neurology, University of British Columbia, Vancouver, BC, Canada
Synopsis
Parkinson’s Disease is not normally associated with white matter changes in clinical MRI scans. Myelin water imaging was used to probe the white matter tissue integrity of mildly affected Parkinson’s Disease patients. Partial Least Squares regression was employed to find a multivariate relation between myelin water fraction along different white matter tracts and motor performance scores. We found association between myelin water fraction and rigidity, bradykinesia and tremor scores, linking changes in white matter tissue integrity to Parkinson’s Disease severity in motor scores.
Purpose
Obvious WM changes, presumably due to small vessel disease, are associated with dementia and cognitive deficits in subjects who also have PD. However, despite widespread motor and cognitive deficits in Parkinson’s Disease, standard clinical MRI scans do not usually detect white matter (WM) changes. More recent studies using diffusion tensor imaging (DTI) have detected microstructural changes in the WM which are associated with cognitive deficits 1,2. On the other hand, studies relating WM changes with motor impairments in PD are scarce. Comorbid WM hyperintensities may be associated with bradykinesia, tremor and rigidity, but not with gait impairments3. Additionally, a study by Lee et al4 found that severity of WM hyperintensities was associated with gait and postural impairments, speech, facial expression, bradykinesia and rigidity, but not with tremor. Here ,we used an advanced and quantitative MRI technique called myelin water imaging (MWI) to explore the relations between microstructural changes of the WM and motor scores in mildly affected PD subjects.Methods
Thirty-one subjects (23 male, 8 female) with mean age of 65.0 ± 6.2 years participated in this study. All subjects underwent a MRI scanning session as well as a cognitive and motor performance examination, assessed by the Montreal Cognitive Assessment (MoCA) and the Unified Parkinsons Disease Rating Scale (UPDRS), respectively. All MR experiments were performed on a 3.0 T MR scanner (Achieva 3.0T, Philips Medical Systems, Netherlands) using an eight channel head coil. The MWI sequence was a gradient and spin echo (GRASE) 3D technique with 32 echoes at TE=10ms, TR=1000ms with a field of view (FOV) of 230cm x 192cm x 100cm with 20 slices collected at 5mm thickness and reconstructed as 40 slices with 2.5mm thickness. The multi-echo GRASE sequence was analyzed as previously described 5, resulting in one MWF map per subject. Twenty ROIs from the JHU MNI standard template were non-linearly registered to each subject’s first echo image using FSL FNIRT 6 and the average MWF per ROI was computed, resulting in 20 bilateral, average MWF indices across the WM for each individual subject. We focused on the UPDRS sub-scores associated with rigidity, bradykinesia, and tremor. We generated different variables by summing all scores in their respective category while keeping them separated in left and right side measures. Lastly, we included the subjects' age and MoCA score in the clinical indices to account for potential age or cognitive effects. Together with the imaging data, this resulted in two sets of multivariate parameters per subject. Partial Least Squares (PLS) was employed to find a complex relation between disease severity scores (UPDRS sub-scores for rigidity, bradykinesia, and tremor) and WM indices of myelin content across the brain. The number of latent variables (LVs) were chosen using the predicted residual estimated sum of squares (PRESS) 7. For this, the number of LVs was increased until the value of PRESS no longer showed improvement. Following this, the clinical scores were estimated based on the PLS regression using the WM indices. To ensure robustness of the estimation we performed leave-one-out cross validation (LOOCV) during the PLS regression. Note that due to the exploratory approach, we did not attempt to predict the UPDRS from the training set during LOOCV, but rather tested the robustness of the estimations across our data sample.Results
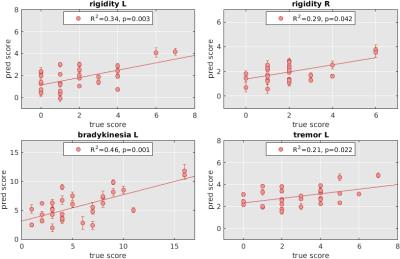
A significant association between MWF and clinical motor indices was detected when controlling for age and cognitive scores. Estimated clinical scores based on WM integrity yielded a moderate estimation power ranging from R2 of 0.21 to R2 of 0.46 across the considered motor sub scores, see Figure 1. Only significant estimation could be performed for rigidity in left and right hand side measures, bradykinesia in left side, and tremor in the left side.Discussion
Our results suggest an association between WM microstructure, quantified by MWF and PD motor severity as rated using UPDRS. Further, we have shown that even in the early stages of PD with no apparent WM alterations, WM microstructure is sensitive to disease severity. Estimations that were only moderately accurate may be due to fact that the cohort study was only mildly affected.Conclusion
WM tissue alterations can be detected in PD and relate to worsening of motor function. MWI can provide complementary information to current techniques to assess the WM of Parkinson’s Disease subjects.Acknowledgements
No acknowledgement found.References
1. Theilmann RJ, Reed JD, Song DD, et al.: White-Matter Changes Correlate with Cognitive Functioning in Parkinson’s Disease. Front Neurol 2013; 4:37.2. Melzer TR, Watts R, MacAskill MR, et al.: White matter microstructure deteriorates across cognitive stages in Parkinson disease. Neurology 2013; 80:1841–9. 3. Sohn YH, Kim JS: The influence of white matter hyperintensities on the clinical features of Parkinson’s disease. Yonsei Med J 1998; 39:50–5. 4. Lee S-J, Kim J-S, Lee K-S, et al.: The severity of leukoaraiosis correlates with the clinical phenotype of Parkinson’s disease. Arch Gerontol Geriatr 2009; 49:255–259. 5. Prasloski T, Mädler B, Xiang QS, MacKay A, Jones C: Applications of stimulated echo correction to multicomponent T2 analysis. Magn Reson Med 2012; 67:1803–1814. 6. Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM: FSL. Neuroimage 2012; 62:782–90. 7. Krishnan A, Williams LJ, McIntosh AR, Abdi H: Partial Least Squares (PLS) methods for neuroimaging: A tutorial and review. Neuroimage 2011; 56:455–475.
Figures