2477
Environmental paraquat exposure and HFE genetics as factors in the development of Parkinson’s disease1Neurosurgery, The Pennsylvania State University - College of Medicine, Hershey, PA, United States, 2Radiology, The Pennsylvania State University - College of Medicine, Hershey, PA, United States, 3Neural and Behavioral Sciences, The Pennsylvania State University - College of Medicine, Hershey, PA, United States
Synopsis
This work demonstrates a decrease in R2 within the ventral-nigral region of WT-HFE mice given paraquat injections. Furthermore, the decrease in R2 relaxation rate is not observed in the H67D-HFE mutation animals. This is the first demonstration that HFE mutations may be associated with a preservation of cellular loss in the substantia nigra and ventral-midbrain of Parkinson’s disease model animals.
Introduction:
The herbicide paraquat (1, 1’-dimethyl-4, 4’-bipyridinium) is robustly used in agricultural areas and it has been established that rural community residents are at greater risk of developing Parkinson’s Disease (PD) (1,2). This provides strong evidence of the neurotoxic effect of paraquat in the development of PD. While the precise action of paraquat is largely unknown, it has been reported that a single injection of paraquat results in increased iron uptake, the production of superoxide radicals within, and pronounced dopaminergic cell loss in the substantia nigra of treated mice (3,4). We have previously demonstrated that the H76D-HFE knock-in mouse, analogous to the H63D mutation in humans, exhibits altered iron homeostasis, that both the H67D mouse and the highly prevalent H63D-HFE human polymorphism contributes to cortical white matter abnormalities (5), and that HFE mutations are related to the neurodegenerative disease process (6). It remains unknown how the HFE mutation relates to Parkinson’s disease, as such we evaluated how the HFE mutation and paraquat exposure contribute to Parkinson’s.Methods:
A total of 31 four-month old C57BL/6J mice were utilized in this study design: 16 HFE-wildtype (WT) and 15 HFE-H67D/H67D knock-in animals. Mice were housed under a standard light cycle, fed ad libitum, and cared for in accordance with IACUC policies. Mice were imaged on a Bruker 7T system at four-months of age with baseline behavioral and MR metrics taken prior to injection with paraquat (N=16) or saline (N=15) (15 mg/kg, injected i.p,). Two subsequent injections, for a total of three, were given at weekly intervals over a four week period following by MR imaging, behavioral metrics, and animal sacrifice. Relaxation maps were computed from the nine echo spin-echo dataset using in-house software (qMRI). SPMMouse within the SPM8 suite was used to register individual datasets, normalize to a template brain, and conduct statistical analyses.Results:
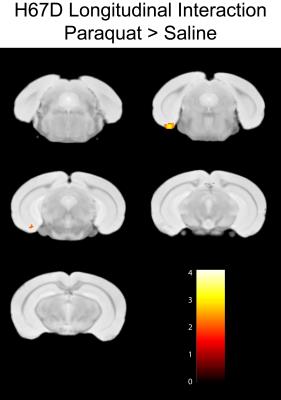
WT-HFE mice given paraquat injections demonstrate a group based increase in R2 rate within the substantia nigra, zona incerta, and ventral midbrain of paraquat injected animals compared to WT-HFE controls animals given a saline injection (Fig. 1). The interaction map demonstrate regions where an increase in R2 is found in the paraquat animals over the four week period compared to saline controls. The same reduction in R2 parametrics is not observed in the H67D-HFE knock-in animals compared to H67D-HFE controls, demonstrating a different trajectory in the H67D-HFE carriers.Discussion:
This MR metric data suggests that paraquat has a marked effect upon cellular structure in animals given paraquat IP injections. The increase in R2 within the nigral-ventral midbrain region is hypothesized to be related to a loss of cellularity within this region; associated with increased inflammation and cellular loss. The trajectory of the H67D-HFE animals given paraquat injections differs from the WT animals. There is evidence to support a preservation in brain structures associated with HFE genetic mutations in patients with neurodegenerative disease (7,8). Our concurrent research into HFE genetics in neurodegenerative disease also demonstrates a preservation of brain structure in Alzheimer’s HFE carriers.Conclusion
This work demonstrates a
decrease in R2 within the ventral-nigral region of WT-HFE mice given paraquat
injections. Furthermore, the decrease in
R2 relaxation rate is not observed in the H67D-HFE mutation animals. This is the first demonstration that the H63D-HFE
mutation may be associated with a preservation of cellular loss in the
substantia nigra and ventral-midbrain of Parkinson’s disease animals. Further work
will use ongoing diffusion and quantitative susceptibility metrics as well as concurrent
histological methods to determine the cellular cause of the MRI parametrics
changes associated with HFE genetics.Acknowledgements
No acknowledgement found.References
1. Wang A, Costello S, Cockburn M, Zhang X, Bronstein J, Ritz B. Parkinson's disease risk from ambient exposure to pesticides. Eur J Epidemiol 2011;26(7):547-555.
2. Thiruchelvam M, Brockel BJ, Richfield EK, Baggs RB, Cory-Slechta DA. Potentiated and preferential effects of combined paraquat and maneb on nigrostriatal dopamine systems: environmental risk factors for Parkinson's disease? Brain Res 2000;873(2):225-234.
3. Yin L, Lu L, Prasad K, Richfield EK, Unger EL, Xu J, Jones BC. Genetic-based, differential susceptibility to paraquat neurotoxicity in mice. Neurotoxicol Teratol 2011;33(3):415-421.
4. Cocheme HM, Murphy MP. Complex I is the major site of mitochondrial superoxide production by paraquat. J Biol Chem 2008;283(4):1786-1798.
5. Meadowcroft MD, Wang J, Purnell CJ, Peters DG, Eslinger PJ, Neely EB, Gill DJ, Vasavada M, Ali-Rahmani F, Yang QX, Connor JR. Reduced white matter MRI transverse relaxation rate in cognitively normal H63D-HFE human carriers and H67D-HFE mice. Brain Imaging Behav 2015.
6. Ali-Rahmani F, Schengrund CL, Connor JR. HFE gene variants, iron, and lipids: a novel connection in Alzheimer's disease. Front Pharmacol 2014;5:165.
7. Wang ZX, Wan Y, Tan L, Liu J, Wang HF, Sun FR, Tan MS, Tan CC, Jiang T, Yu JT. Genetic Association of HLA Gene Variants with MRI Brain Structure in Alzheimer's Disease. Mol Neurobiol 2016.
8. Johnstone DM, Graham RM, Trinder D, Riveros C, Olynyk JK, Scott RJ, Moscato P, Milward EA. Changes in brain transcripts related to Alzheimer's disease in a model of HFE hemochromatosis are not consistent with increased Alzheimer's disease risk. J Alzheimers Dis 2012;30(4):791-803.
Figures