2473
Peculiarities of brain activation during dominant hand tactile perception in lateralized Parkinson disease1Human and Animal Physiology, Taras Shevchenko National University of Kyiv, Kyiv, Ukraine, 2Radiology, Medical Clinic BORIS, Kyiv, Ukraine, 3Department of extrapyramidal disorders, D. F. Chebotarev Institute of Herontology, Kiev, Ukraine
Synopsis
Parkinson's disease (PD) in mostly presented with asymmetrical motor symptoms. Disturbed sensorimotor integration and decrease of somatosensory cortex activation was previously shown. We analyzed brain activation and connectivity during the unilateral tactile stimulation in primary and non-primary hand lateralized PD patients. We have demonstrated steady contralateral S1 activation in PD. Primary hand tactile stimulation in primary hand lateralized PD patients evokes activation of primary and associative sensory, motor and executive nodes of the cortex. Mirror neuron system was activated in primary hand stimulation in PD patients. Tactile stimuli processing evokes increased connectivity of globus pallidus, premotor, prefrontal and parietal cortex.
Introduction
Parkinson's disease (PD) in mostly presented with asymmetrical motor symptoms1. Association exists between dominant hand and side of the initial motor symptom in PD2. Right-hand PD symptoms onset is related to the increased symptoms severity and shorter ambulatory disease survival3. Previously we have shown that primary sided symptoms influence greater stimulus-to-movement switching of the brain networks4. Decrease of movement related lateralization of brain activation was earlier shown5. Disturbed sensorimotor integration4,6 and elevations in sensory threshold were shown for PD patients6. Several studies claim that during tactile stimulation patients with PD had less activity in bilateral sensorimotor cortical areas7,8,9. We propose brain activation and connectivity analysis during the unilateral tactile stimulation in primary and non-primary hand motor symptoms lateralized PD patients for the motor PD symptom asymmetry impact study onto the somatosensory processing in PD.Methods
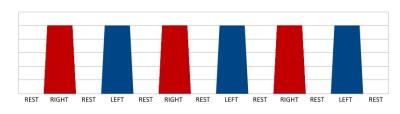
Three groups of right-handed subjects (G1, G2, G3) were studied by fMRI with 1.5T Signa HDx (GE, USA). G1 consisted of 7 healthy subjects (4F, 51-83 y.o.). G2 consisted of 6 non-demented (MoCA 25-27) PD patients with left-side (non-primary hand) motor symptoms (2F, 53-74 y.o.). G3 consisted of 6 non-demented (MoCA 25-30) PD patients with right-side (primary hand) motor symptoms (2F, 56-74 y.o.). For G2/G3 disease duration was 1-4 years, Hoen-Yahr scale 2.5-3. Simple reaction time in G1/G2/G3=302/352/328 ms. Simple index finger tactile stimulation successively alternating for right and left hand was used for somatosensory fMRI activation (Fig.1). Stimulation was made by the plastic wheel. Scanning session time was 4 min 25 s, 6 blocks of activation (3 for right hand and 3 for left in alternating way) were acquired. Three estimation variables (EV) were generated. EV1 corresponded to the index finger of the right-hand stimulation, EV2 corresponded to the index finger of the left-hand stimulation, EV3 corresponded to the index finger either of the right or left-hand stimulation. EV1 was orthogonalized with EV2 and vice versa. EV3 was orthogonalized with EV1 and EV2. As the movement execution supposed predictable BOLD signal oscillation, estimated frequency for BOLD fluctuation for EV3 was ƒ1=2.31x10-2 Hz. Single shot GE-EPI sequence was used for BOLD imaging (TR/TE=3000/71 ms, voxel size=4x4x5 mm). Anatomical images were acquired with FSPGR sequence (TR/TE=11.6/5.2 ms, TI=450 ms, voxel size=1x1x1.5 mm). FMRI data processing was carried out using GLM (FEAT) and ICA (MELODIC) based software from FSL (Oxford, GB). Standard FSL pre-processing was done. Single subject and group GLM and ICA analyses were done. ICA correlation analysis with GLM data was done using a built-in F-test. The frequency spectrum of BOLD signal fluctuations was analyzed for ICA components.Results and Discussion
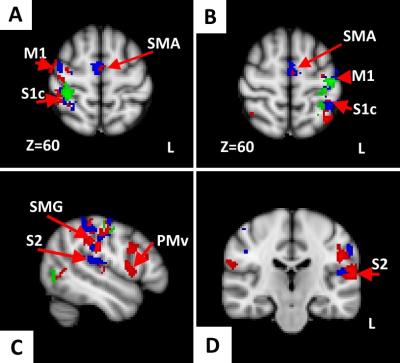
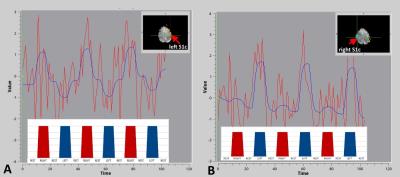
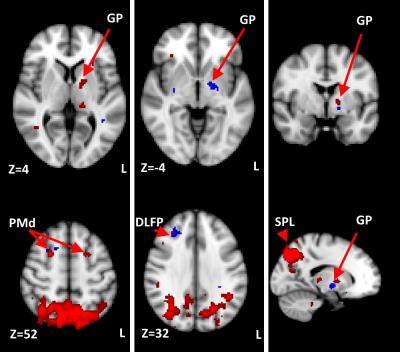
GLM and ICA-based fMRI data analysis in G1/G2/G3 revealed activation of contralateral postcentral gyrus (primary somatosensory cortex, S1c) at the level of ‘hand knob’ (Z=60, MNI152), (G1, G2, G3) (Fig.2, Fig 3.). In several G2/G3 patients activation also was found in ipsilateral S1i, precentral gyrus, at the level of ‘hand knob’ (primary motor area, M1) and supplementary motor area (SMA). In G2/G3 primary (right) hand stimulation evoked more pronounced activation in S1c, S1i, M1, contralateral superior parietal lobule, supramarginal gyrus (SMG). In G3 activation of contralateral ventral premotor area was found during each hand stimulation. In G2/G3 activation of inferior part of pre/postcentral gyri located mainly in the lateral sulcus (secondary somatosensory cortex, S2) was found during the both right and left-hand stimulation. EV3 related activation of SMA was found in G2/G3. ICA analysis revealed increased functional connectivity in the regions of left globus pallidus (GP), right dorsal premotor cortex (PMd), right dorsolateral prefrontal cortex (DLPF) in G2 and bilateral PMd, right DLPF, bilateral superior parietal lobuli (SPL) in G3 (Fig.4). Main frequency of the ICA component spectrum for described networks was ν1=2.31x10-2 Hz=ƒ1.
Thus, unlike several other authors7,8,9, we have demonstrated steady contralateral S1 activation in PD (Fig.3). Unlike healthy controls, PD patients demonstrated activation of S2, SMA, SMG and PMv. SMG and PMv are known to form mirror neuron system, which seems to participate in compensatory mechanism in PD. Increased functional connectivity of the GP, PMd, DLPF might reveal tactile-evoked motion suppression in PD, as the DLPF known to control the reaction to the external stimulus10 and executive functions11. Lateralization of somatosensory-related brain activation was decreased in PD.
Conclusions
Primary somatosensory cortex remains steadily active during tactile stimulation in PD, while dominant hand tactile stimulation in dominant hand lateralized PD patients also evoke activation of primary and associative sensory, motor and executive nodes of the cortex. Tactile stimuli processing evokes increased connectivity of globus pallidus, premotor, prefrontal and parietal cortex.Acknowledgements
Vasiliy Vakorin, Simon Fraser University, Burnaby, Canada.
Maria Guidi, Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany.
References
1. Scharoun SM, Bryden PJ, Sage MD, Almeida QJ, Roy EA. The Influence of Parkinson’s Disease Motor Symptom Asymmetry on Hand Performance: An Examination of the Grooved Pegboard Task. Parkinson’s Disease. 2015;2015:307474. doi:10.1155/2015/307474.
2. Barrett MJ, Wylie SA, Harrison MB, Wooten GF. Handedness and motor symptom asymmetry in Parkinson’s disease. Journal of neurology, neurosurgery, and psychiatry. 2011;82(10):1122-1124. doi:10.1136/jnnp.2010.209783.
3. Munhoz RP, Espay AJ, Morgante F, et al. Long-duration Parkinson's disease: role of lateralization of motor features. Parkinsonism & Related Disorders. 2013;19(1):77–80. doi: 10.1016/j.parkreldis.2012.07.008.
4. Omelchenko O, Rozhkova Z, Karaban I Parkinson’s disease laterality impact onto the default mode network deactivation by the audio-motor transformation. Proceedings of ISMRM 2016, #3793.
5. Wu T, Hou Y, Hallett M, Zhang J, Chan P. Lateralization of brain activity pattern during unilateral movement in Parkinson's disease. Hum Brain Mapp. 2015 May;36(5):1878-91. doi: 10.1002/hbm.22743. PubMed PMID: 25644527.
6. Conte A, Khan N, Defazio G, Rothwell JC, Berardelli A. Pathophysiology of somatosensory abnormalities in Parkinson disease. Nat Rev Neurol. 2013 Dec;9(12):687-97. doi: 10.1038/nrneurol.2013.224. Review. PubMed PMID: 24217516
7. Cao H, Xu X., Zhao Y, Long D. & Zhang. M. Altered brain activation and connectivity in early Parkinson disease tactile perception. AJNR Am. J. Neuroradiol. 32, 1969–1974 (2011).
8. Weder B, Azari NP, Knorr U, et al . Disturbed functional brain interactions underlying deficient tactile object discrimination in Parkinson's disease. 2000;11:131–45.
9. Boecker H, Ceballos-Baumann A, Bartenstein P, et al. Sensory processing in Parkinson's and Huntington's disease: investigations with 3D H(2)(15)O-PET. Brain 1999;122(Pt 9):1651–65
10. Lee TG; Blumenfeld RS; d'Esposito M. (2013). "Disruption of Dorsolateral but Not Ventrolateral Prefrontal Cortex Improves Unconscious Perceptual Memories". Journal of Neuroscience. 33 (32): 13233–7. doi:10.1523/JNEUROSCI.5652-12.2013. PMID 23926275.
11. Goldman-Rakic Patricia S. (1995). "Architecture of the Prefrontal Cortex and the Central Executive". Annals of the New York Academy of Sciences. 769: 71–83. doi:10.1111/j.1749-6632.1995.tb38132.x. PMID 8595045.
Figures