2468
Regional impaired cerebrovascular reactivity in migraine with and without aura in the interictal state: A pilot fMRI study1Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Charlestown, MA, United States, 2Biogen Inc., Cambridge, MA, United States, 3Division of Neuroradiology, Department of Radiology, Massachusetts General Hospital, Boston, MA, United States, 4Department of Neurology, Massachusetts General Hospital, Boston, MA, United States
Synopsis
Ischemia within the posterior circulation has been proposed as a primary mechanism for migraine. Though, in-vivo studies have yet to fully elucidate the underpinnings of this mechanism. In the current study, angiography via time of flight (ToF) MR was used to identify potential structural deficits within the posterior circulation and hypercapnic BOLD fMRI was used to detect functional vascular defects by quantifying cerebral vascular reactivity (CVR). All three MoA subjects demonstrated a negative correlation in BOLD signal within the red nuclei during CO2 challenge whereas the three MA patients demonstrated CVR within the red nuclei that was similar to that of the control subjects. ToF MR angiography images from all MoA subjects showed hypoplasia of bilateral posterior communicating arteries (PCoA) in proximity of the circle of Willis. In contrast only one out of the three MA subjects showed PCoA hypoplasia on ToF images. Our findings of hypoplasia of posterior communicating arteries combined with abnormal CVR responses within the red nuclei provide both structural and functional evidence for differential vascular defects in the migraine samples studied. We suggest that the identified vascular deficits to impose vulnerability in midbrain blood supply that may likely contribute to the migraine pathophysiology.
Introduction
Converging
evidence supports a role for vascular
dysfunction in migraine pathophysiology. Specifically, ischemia within the posterior
circulation has been proposed as a primary mechanism for migraine 1, 2. Though, in-vivo studies have yet to fully elucidate the
underpinnings of this mechanism. The
current study employed both structural and functional cerebral imaging techniques
to probe for vascular deficits in migraine patients. Angiography via time of
flight (ToF) MR was used to identify potential structural deficits within the
posterior circulation and hypercapnic BOLD fMRI was used to detect functional vascular
defects by quantifying cerebral vascular reactivity (CVR). Hypercapnic fMRI has proven to be sensitive
and specific in identifying differential CVR effects in both magnitude
(amplitude) and temporal domains in several vasculopathies 3-6.
By utilizing this multimodal imaging approach we sought to identify candidate
structural and functional vulnerabilities within the posterior circulation of
migraine patients.Subjects and Methods
Participants: Six
right-handed patients with episodic, Grade 2 migraine severity and five
headache-free controls (9 males, 2 females, aged 22-48 years) were studied. Three of patients had
migraine with aura (MA) and 3 had
migraine without aura (MoA). All patients had disease duration >1year, and the average frequency of
migraine attacks was 4 attacks per month. MRI
was performed during the inter-ictal phase on a 3 Tesla Siemens scanner. The
'inter-ictal' phase was defined as a migraine-free period ≥ 72 hours prior or ≥ 72 hours following a migraine episode. Methods: Whole brain MRI datasets acquired in each subject included:
1) a ToF angiography sequence to identify the patency
and abnormalities within the major intracranial cerebral arteries; 2) a gradient-echo,
echo planar BOLD sequence (TR=1450ms, TE=30ms, FOV=220mm, matrix=64´64, thickness=5mm) during hypercapnic challenge. Prior to hypercapnic challenge the resting end-tidal
carbon dioxide (PCO2) was assessed in each subject via calibrated
capnograph. The fraction of inspired carbon dioxide was adjusted to produce
steady-state conditions of normocapnia and mild hypercapnia (4-8 mmHg above the
subject’s resting PCO2). The paradigm consisted of alternating periods
of normocapnia (~60 sec) and hypercapnia (~30 sec). An MRI-compatible breathing
circuit maintained each subject’s PCO2 within ±1-2
mmHg of target PCO2 7, 8.
Data analysis: MR
angiographic images acquired with ToF sequence were interpreted by a
neuroradiologist for the patency of posterior circulation. Analyses of BOLD-fMRI data were performed
with Analysis of Functional NeuroImage (AFNI) software. The time-series of PCO2
data was corrected for the time lag due to dead space in the sample line as
well as the delay of brain responses using cross-correlation with simultaneously
recorded respiratory waveforms. CVR maps were derived using multiple regression
that incorporated the time-series of percent BOLD signal changes and the
associated time-series of PCO2 changes over the course of each scan. Analyses of statistical parametric maps were corrected
at threshold of p<0.05.
Results
Hypercapnic
challenge revealed group differences in cerebrovascular response. In control
subjects, dynamic changes in PCO2 were associated with strong,
positive correlations in BOLD signal change. In the patients, dynamic changes
in PCO2 were associated with either negative correlations in BOLD
signal change or significant temporal delays. Moreover, the BOLD responses during
hypercapnic challenge were significantly different between MoA and MA patients
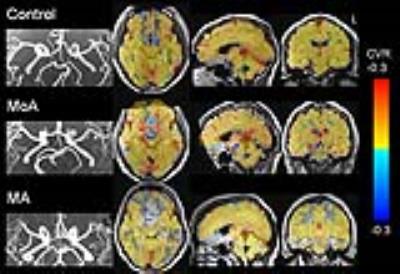
within the red nuclei (Figure 1). All three
MoA subjects demonstrated a negative correlation in BOLD signal within the red nuclei
during CO2 challenge whereas the three MA patients demonstrated CVR
within the red nuclei that was similar to that of the control subjects. The amplitude of CVR within the red nuclei
was found to be positively correlated with the frequency of migraine attacks for
the MoA subjects. In all 3 MoA subjects, ToF MR angiography images showed
hypoplasia of bilateral posterior communicating arteries (PCoA) in proximity of
the circle of Willis. In contrast only one out of the three MA subjects showed PCoA
hypoplasia on ToF images.Discussion
Taken
together, our findings of PCoA hypoplasia combined with abnormal CVR responses within
the red nuclei provide both structural and functional evidence for differential
vascular defects in the migraine samples studied. We suggest that the
identified vascular deficits to impose vulnerability in midbrain blood supply
that may likely contribute to the migraine pathophysiology. Acknowledgements
No acknowledgement found.References
1. Caplan LR. Migraine and vertebrobasilar ischemia. Neurology 1991;41:55-61.
2. Shin DH, Lim TS, Yong SW, et al. Posterior circulation embolism as a potential mechanism for migraine with aura. Cephalalgia 2012;32:497-499.
3. Han JS, Mikulis DJ, Mardimae A, et al. Measurement of cerebrovascular reactivity in pediatric patients with cerebral vasculopathy using blood oxygen level-dependent MRI. Stroke 2011;42:1261-1269.
4. Krainik A, Hund-Georgiadis M, Zysset S, et al. Regional impairment of cerebrovascular reactivity and BOLD signal in adults after stroke. Stroke 2005;36:1146-1152.
5. Mikulis DJ, Krolczyk G, Desal H, et al. Preoperative and postoperative mapping of cerebrovascular reactivity in moyamoya disease by using blood oxygen level-dependent magnetic resonance imaging. J Neurosurg 2005;103:347-355.
6. Ziyeh S, Rick J, Reinhard M, et al. Blood oxygen level-dependent MRI of cerebral CO2 reactivity in severe carotid stenosis and occlusion. Stroke 2005;36:751-756.
7. Banzett RB, Garcia RT, Moosavi SH. Simple contrivance "clamps" end-tidal PCO(2) and PO(2) despite rapid changes in ventilation. J Appl Physiol 2000;88:1597-1600.
8. McKay LC, Evans KC, Frackowiak RS, et al. Neural correlates of voluntary breathing in humans. J Appl Physiol 2003;95:1170-1178.