2465
Assessment of cerebral perfusion autoregulation impairment – An experimental setup to quantify regional cerebral blood flow (CBF) in normal and head down tilt position1Buffalo Neuroimaging Analysis Center, Department of Neurology, Jacobs School of Medicine and Biomedical Sciences, University at Buffalo, The State University of New York, Buffalo, NY, United States, 2Health Sciences Fabrication Shop, Department of Medicine,Jacobs School of Medicine and Biomedical Sciences, University at Buffalo, The State University of New York, Buffalo, NY, United States, 3MRI Clinical and Translational Research Center, Jacobs School of Medicine and Biomedical Sciences, University at Buffalo, The State University of New York, Buffalo, NY, United States, 4Department of Psychiatry,Jacobs School of Medicine and Biomedical Sciences, University at Buffalo, The State University of New York, Buffalo, NY, United States, 5Department of Orthopedics,Jacobs School of Medicine and Biomedical Sciences, University at Buffalo, The State University of New York, Buffalo, NY, United States
Synopsis
Previous studies using Transcranial Doppler have shown that cerebral ischemia, head trauma, and cerebral perfusion pressure are associated with an impairment of the cerebral autoregulation (CA) and alteration of perfusion. However, simulating perfusion changes and quantifying them with higher specificity repetitively has been a challenge in clinics. We propose a clinical experimental setup for an MRI-based head down tilt protocol to study the CA by quantifying perfusion. We demonstrate local perfusion change results in healthy controls and a patient.
Purpose:
Cerebral autoregulation (CA) ensures the constancy of the cerebral blood flow (CBF). Using Transcranial Doppler (TCD), previous studies have shown that cerebral ischemia, head trauma, and cerebral perfusion pressure are associated with an impairment of the CA and alteration of local CBF1-5. These studies motivate the use of CA and CBF measurements as clinical biomarkers for these pathologies. Although TCD is highly sensitive, it lacks specificity and is highly operator-dependent6,7.
Here, we present a clinically applicable experimental setup for MRI-based head-down tilt (HDT) measurements of CA. The HDT experiment assesses the CA by quantifying perfusion at different body positions in one patient with a sub-acute concussion and two healthy controls (HCs).
Method:
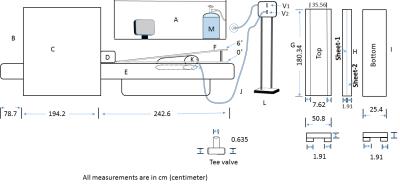
Experimental setup: A primary design objective for the HDT setup was that the experiment could be performed by the MRI technologist. A schematic illustration of the setup is shown in Figure-1, which consisted a special patient table that was placed on vendor’s original patient bed and could be inclined at the foot-end during an MRI session while the patient was inside the magnet. High-density polyethylene (HDPE) sheets (19mm thickness) provided sufficient stability at a minimal added vertical height of the bed at the head-end to use a vendor-provided head coil with limited cable length. An inflatable 42L polyvinylchloride airbag was placed between two HDPE-sheets, which was connected via thermoplastic rubber pipes and a custom pressure valve system (HDPE tee-valves) to a compressed air cylinder in the control room. Opening the valve raised the wedge within 1-2 minutes to 6˚, an angle that was chosen based on physiological studies on CA in HCs17-19,24,23.
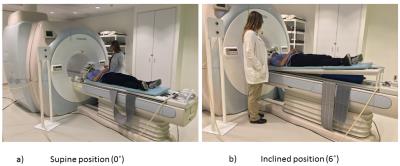
Experiment: The setup was tested in 2 HCs (29 and 38 years; male) and 1 patient (15 years; female) with a post-acute concussion (IRB approved) at 3T (Toshiba Vantage Titan) using a 32 channel brain coil in supine(0˚)-incline(6˚)-supine(0˚) positions. CBF was measured using the ASTAR pulsed ASL (pASL) sequence14,15,16 with a 3D Swirl-SSFP readout (QUIPPSII16;BW=977Hz/px, TE/TR/TI/TTEC=2/2800/2000/800ms, Nsaturation=3, Navg=4, FOV=26x26x10.4cm3, matrix=64X64x13, 15cm tag thickness with 2cm gap, 10cm TEC thickness, TScan=5:22min:sec) and a PDw sequence (TScan=41sec). First, we studied the reproducibility by applying three times ASL (PDw once) in 0° (Figure-2a). To study the temporal dynamics of CBF at 6˚ (Fig.-2b), the airbag was inflated (opening V1 in Figure-1) and immediately the ASL were acquired four times (PDw once). Finally, we emptied the airbag (opening V2; Fig.-2a), and measured CBF recovery dynamics with four ASL at 0°. A high-resolution T1-weighted anatomical scan (TE/TR/TI=3.2/6.2/900ms, FOV=25.6x25.6x19.2cm3, matrix=256x256x192, TScan=5.59min) was acquired before the first ASL sequence for a region-based analysis of CBF maps and localized shimming was applied in each positions.
Data Analysis: ASL and PDw images were reconstructed in absorption-mode from k-space using coil sensitivity maps and registered22 to the T1w images. Voxel-wise division of ASL tag-control images by the PD and application of appropriate scaling factors (T1blood=1650ms26, λ=0.9ml/g27, 98% labeling efficiency16; T1-correction with T1GM=1300ms) yielded the CBF 16. A probabilistic MNI atlas21,28 was applied to determine average CBF values in gray matter (GM), whole brain, white matter, insular GM, parietal lobe GM, occipital lobe GM, and brain stem.
Results:
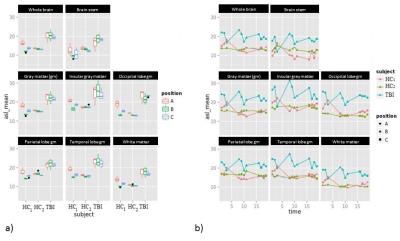
Mean CBF values were significantly different between the three positions (p<0.001) in HCs, with reduced CBF in the 6° position compared to 0° (Fig. 3a). The patient showed an overall increased average CBF compared to controls (Fig.3a,b),and a sharply increasing CBF was noticed immediately following the inclination (scan 4; Fig. 3b; blue), whereas CBF declined in HC1 and remained stable in HC2 (Fig. 3b; yellow/red). The initial CBF increase in the inclined patient was followed by a rapid decline to the baseline in the following three ASL measurements. Upon return to the normal supine position, another sharp increase was observed in the patient.Discussion:
We presented an experimental setup that allows performing HDT experiments in an MRI scanner without repositioning of the subject, as it was required in previous HDT experiments25. While CBF was relatively static in HCs (or decreased at 6°) due to an intact CA, CBF increased in the patient after every position change, followed by a rapid decline (over 15 minutes) to the baseline, in line with a less efficient CA compared to controls. While these findings need to be systematically confirmed in a larger group, the rapid change of CBF in the patient after position changes may allow clinical measurements of the CA with only one ASL scan in each position, limiting scan time and patient discomfort.Conclusion:
The presented HDT-setup sets the foundation for clinically feasible measurements of the CA.Acknowledgements
Research reported in this publication was funded by Toshiba Medical Systems Corporation, the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR001412, and the National Institute of Neurological Disorders & Stroke under Award Number R01NS094444. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.References
1. Bellapart, Judith, and John F. Fraser. "Transcranial Doppler assessment of cerebral autoregulation." Ultrasound in medicine & biology 35.6 (2009): 883-893.
2. Dewitt, L. Dana, and Lawrence R. Wechsler. "Transcranial Doppler." Stroke 19.7 (1988): 915-921.
3. Razumovsky, A. V., et al. "TCD, MRA and MRI in acute cerebral ischemia." Acta neurologica scandinavica 99.1 (1999): 65-76.
4. Sloan, M. A., et al. "Assessment: transcranial Doppler ultrasonography report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology." Neurology 62.9 (2004): 1468-1481.
5. Chan, Kwan-Hon, et al. "The effect of changes in cerebral perfusion pressure upon middle cerebral artery blood flow velocity and jugular bulb venous oxygen saturation after severe brain injury." Journal of neurosurgery 77.1 (1992): 55-61.
6. Sorond, Farzaneh A., et al. "Brain Blood Flow and Velocity Correlations between Magnetic Resonance Imaging and Transcranial Doppler Sonography." Journal of Ultrasound in Medicine 29.7 (2010): 1017-1022.
7. Razumovsky, A. V., Gillard, J. H., Bryan, R. N., Hanley, D. F. and Oppenheimer, S. M. (1999), TCD, MRA and MRI in acute cerebral ischemia. Acta Neurologica Scandinavica, 99: 65–76.
8. Marcel J.H. Aries, Jan W. Elting, Jacques De Keyser, Berry P.H. Kremer and Patrick C.A.J. Vroomen, Cerebral Autoregulation in Stroke: A Review of Transcranial Doppler Studies. Stroke, November 2010, Volume 41, Issue 11.
9. Paulson, O. B., S. Strandgaard, and L. Edvinsson. "Cerebral autoregulation." Cerebrovascular and brain metabolism reviews 2.2 (1989): 161-192.
10. Reivich, M., W. J. S. Marshall, and N. Kassell. "Loss of autoregulation produced by cerebral trauma." Cerebral blood flow. Springer Berlin Heidelberg, 1969. 205-208.
11. Strandgaard, Svend. "Autoregulation of cerebral circulation in hypertension." (1978).
12. Lassen, Niels A. "Cerebral blood flow and oxygen consumption in man." Physiological reviews 39.2 (1959): 183-238.
13. Laura M. Parkes,* Waqar Rashid, Declan T. Chard, and Paul S. Tofts, Normal Cerebral Perfusion Measurements Using Arterial Spin Labeling: Reproducibility, Stability, and Age and Gender Effects, Magnetic Resonance in Medicine 51:736–743 (2004)
14. Kimura, Tokunori. "MRI apparatus, flow quantification apparatus, and flow quantification method for ASL imaging." U.S. Patent No. 7,627,360. 1 Dec. 2009
15. Tokunori Kimura, Kabushiki Kaisha Toshiba, “United States patent: 6564080 B1- MR imaging on ASL technique”, May 13, 2003.
16. Wong, Eric C., Richard B. Buxton, and Lawrence R. Frank. "Quantitative imaging of perfusion using a single subtraction (QUIPSS and QUIPSS II)." Magnetic resonance in medicine 39.5 (1998): 702-708.
17. Olszowka, A. J., et al. "Cardiac output: a view from Buffalo." European journal of applied physiology 90.3-4 (2003): 292-304.
18. Olszowka AJ, BE Shykoff, DR Pendergast, LE Farhi. Revised Single-Breath Method for Determination of Cardiac Output. Respir Physiol, 247:1-11, 2003.
19. Bednarczyk EM, DP Pendergast, DS Wack, A Lockwood. A pilot study of cerebral blood flow (CBF) under physiological simulations of altered gravity. American Society for Clinical Pharmacology and Therapeutics. 2002
20. Marshall-Goebel, Karina, et al. "Effects of Short-Term Exposure to Head Down Tilt on Cerebral Hemodynamics: A Prospective Evaluation of a Space-Flight Analog using Phase Contrast MRI." Journal of Applied Physiology (2016): jap-00841.
21. VS Fonov, AC Evans, K Botteron, CR Almli, RC McKinstry, DL Collins and BDCG, Unbiased average age-appropriate atlases for pediatric studies, NeuroImage,Volume 54, Issue 1, January 2011, ISSN 1053–8119
22. Avants, Brian B., Nick Tustison, and Gang Song. "Advanced normalization tools (ANTS)." Insight J 2 (2009): 1-35.
23. Kawai, Y., et al. "Cerebral blood flow velocity in humans exposed to 24 h of head-down tilt." Journal of Applied Physiology 74.6 (1993): 3046-3051.
24. Kurihara, Koichi, Azusa Kikukawa, and Asao Kobayashi. "Cerebral oxygenation monitor during head-up and-down tilt using near-infrared spatially resolved spectroscopy." Clinical physiology and functional imaging 23.4 (2003): 177-181.
25. Pavilla, Aude, et al. "Absolute and regional cerebral perfusion assessment feasibility in head-down position with arterial spin-labeling magnetic resonance. A preliminary report on healthy subjects." Journal of Neuroradiology (2016)
26. Lu H, Clingman C, Golay X, van Zijl PC. Determining the longitudi- nal relaxation time (T1) of blood at 3.0 Tesla. Magn Reson Med 2004; 52:679–682
27. Herscovitch P, Raichle ME. What is the correct value for the brain— blood partition coefficient for water? J Cereb Blood Flow Metab 1985; 5:65–69
28. VS Fonov, AC Evans, RC McKinstry, CR Almli and DL Collins, Unbiased nonlinear average age-appropriate brain templates from birth to adulthood, NeuroImage, Volume 47, Supplement 1, July 2009, Page S102 Organization for Human Brain Mapping 2009 Annual Meeting
Figures