2464
Feasibility of highly accelerated Parallel Imaging of whole brain and neck vessel wall using a high SNR 32-channel coil on 3T1Shenzhen Institutes of Advanced Technology, Shenzhen, People's Republic of China
Synopsis
Three dimensional whole brain and neck vessel wall imaging of high resolution facilities the imaging of intracranial and extracranial arteries in stroke patients. However, its clinical usage is hindered by long scan time. Using a 32-channel head and neck coil system specially designed for high SNR, this work investigated the feasibility of highly accelerated parallel imaging equipped with optimally selected uniform subsampling pattern based on 3D G-factor calculated from separate calibration data and joint sparsity based denoising at acceleration factor of 6.
Purpose
High resolution three dimensional imaging of arterial wall with whole brain and neck coverage allows the examination of intracranial and extracranial arteries simultaneously to detect atherosclerotic plaques in stroke patients 1. However, simultaneous large spatial coverage and high resolution leads to impractical long scan time. Reduced sampling such as Parallel Imaging and Compressed Sensing can shorten the scan time, but typically with the maximum acceleration factor less than 4 due to the limitation of signal-to-noise ratio (SNR). Based on a 32-channel head and neck coil specially designed for high SNR 2, this work investigated the feasibility of highly accelerated parallel imaging with optimally selected 2D uniform subsampling and joint sparsity based denoising to shorten scan time.Method
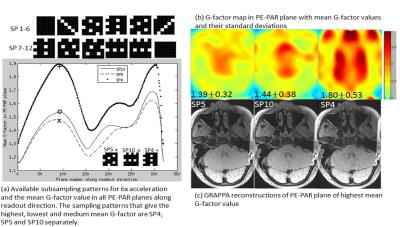
A successful parallel imaging relies on adopted receiver coil, subsampling pattern and reconstruction algorithm. Recently, a 32-channel receiver coil aimed at promoting high SNR was proposed specifically for whole brain and neck vessel wall imaging 2. To maximally utilize the spatial encoding power of this coil, the optimal subsampling pattern given an acceleration factor should be determined. Firstly, this work proposed to select the optimal subsampling pattern from all available GRAPPA and CAIPIRINHA patterns 4 based on the 3D G-factor maps calculated using separate ACS data 3. 6X acceleration with all subsampling patterns were simulated and reconstructed by GRAPPA. Noise behavior in these reconstructions were compared to verify the selection of optimal subsampling pattern. Secondly, the Conjugate Gradient based L1-SPIRiT and GRAPPA algorithms were employed on 6X prospectively accelerated data with the selected optimal trajectory to demonstrate the necessity of joint sparsity prior on suppressing amplified noise 5. Specifically, the 3D recon was decomposed into multiple 2D reconstructions along readout direction for parallel computing 6. Thirdly, the L1SPIRiT reconstructions on that 6X accelerated data was compared with 4X accelerated GRAPPA reconstructions to exhibit the feasibility of highly accelerated parallel Imaging of whole brain and neck vessel wall.Experiment
The IRB approved study was performed on a 3T Siemens Tim Trio MRI system. T1w-SPACE with flip down pulse 1 was used. Firstly, a separate calibration data with size of 64x24x24 (RO x PE x PAR) was acquired for calculating 3D G-factor maps with a fixed GRAPPA kernel size of 5x4x4. The optimal selection was verified on a fully sampled data covering whole brain and neck acquired from one volunteer at an isotropic resolution of 0.62 mm in 22 min. Imaging parameters were: TE/TR = 12/800ms, ETL=41, matrix size = 320x320x208, FOV = 200x200x130 mm3. Secondly, the optimal subsampling pattern for 6X and 4X accelerations were implemented into the T1w-SPACE sequence. Accelerated data was prospectively acquired from another volunteer with imaging parameters: TE/TR = 13/1000ms, matrix size = 320x298x240, FOV = 212x189x158 mm3, and isotropic resolution = 0.66 mm.Results
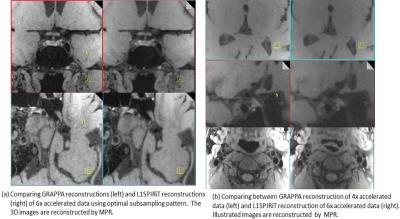
The selection of optimal subsampling patterns using 3D G-factor maps from separate ACS data before imaging was illustrated in Figure 1. For the coil set we used and 6X acceleration, the subsampling patterns that gave the highest and lowest mean G-factor were SP4 and SP5 as shown in Fig 1(a, b). The G-factor of commonly used parallel imaging sampling patterns SP10 was also illustrated. Fig 1(c) demonstrated that the noise behaviors in images reconstructed from simulated subsampled data were consistent with the pre-calculated G-factor maps. Figure 2(a) compared GRAPPA and L1SPIRiT reconstructions of 6X accelerated data using the optimal subsampling pattern. No residual aliasing artifacts were observed in both reconstructions. Amplified noise in GRAPPA reconstructions were significantly suppressed by L1SPIRiT, and benefited the depiction of vessel walls. L1SPIRiT reconstructions of 6X accelerated data were also compared with GRAPPA reconstructions of 4X accelerated data. We can see comparable image quality in the extracranial arteries due to high SNR from the neck coil, while signal degradation in the intracranial arteries due to moderate SNR from the head coil. Moreover, the noise behaviors were similar in 4X GRAPPA reconstructions and 6X L1SPIRiT reconstructions.Discussion and Conclusion
This study demonstrated selecting an optimal subsampling pattern for given coil set before scanning based on 3D G-factor maps calculated from separate calibration data. In vivo results showed that joint-sparse denoising could suppress noise amplified by parallel imaging efficiently and benefited the depiction of vessel walls at a high acceleration factor of 6. Further work will improve the coil design for better parallel imaging performance. Also, 3D parallel imaging and 3D sparse priors can be employed to replace current 2D reconstruction.Acknowledgements
Grant support: China NSFC 61471350, the Natural Science Foundation of Guangdong 2015A020214019, the Basic Research Program of Shenzhen JCYJ20140610151856736.References
[1] Zhang L, Zhang N, Wu J, Zhang L, Huang Y, Liu X, and Chung Y. High resolution three dimensional intracranial arterial wall imaging at 3T using T1 weighted SPACE. Magnetic Resonance Imaging, 2015; 33(9):1026-1034.
[2] Hu X, Li Y, Zhang L, Zhang X, Liu X, and Chung Y. A 32-channel coil system for MR vessel wall imaging of intracranial and extracranial arteries at 3T. Magnetic Resonance Imaging, 2016; in press.
[3] Breuer FA, Kannengiesser SAR, Blaimer M, Seiberlich N, Jakob PM, and Griswold MA. General Formulation for Quantitative G-factor Calculation in GRAPPA Reconstruction. Magnetic Resonance in Medicine, 2009; 62:739-746.
[4] Breuer FA, Blaimer M, Heidemann RM, Mueller MF, Griswold MA, and Jakob PM. Controlled Aliasing in Volumetric Parallel Imaging (2D CAIPIRINHA). Magnetic Resonance in Medicine, 2006; 55:549-556.
[5] Lustig M and Pauly JM. SPIRiT: Iterative Self-consistent Parallel Imaging Reconstruction From Arbitrary k-Space. Magnetic Resonance in Medicine, 2010; 64:457-471.
[6] Murphy M, Alley M, Demmel J, Keutzer K, Vasanawala S, and Lustig M. Fast l1-SPIRiT compressed sensing parallel imaging MRI: scalable parallel implementation and clinically feasible runtime. IEEE Trans. On Medical Imaging, 2012; 31(6):1250-1262.
Figures