2445
Cerebral Blood Flow of CASL Perfusion Imaging to Predict Neurobehavioral Outcome in a Murine Model of Subarachnoid Hemorrhage1Department of Preclinical Evaluation, Institute of Development, Aging and Cancer (IDAC), Tohoku University, Sendai, Japan, 2Research Institute for Brain and Blood Vessels-AKITA, Akita, Japan, 3Department of Nuclear Medicine and Radiology, IDAC, Tohoku University, Sendai, Japan, 4Graduate School of Psychology, Kobe Shoin Women’s University, Kobe, Japan
Synopsis
Early brain injury/ischemia is a recent therapeutic target of subarachnoid hemorrhage (SAH) that contributes to triggering delayed cerebral ischemia (DCI) [1]. However, little is known about the role of cerebral blood flow (CBF) and neurobehavioral profiles at acute stage on functional outcome of the rodent model to simulate clinical severity early after SAH. The present study demonstrated the feasibility of MRI-based CBF measurements using the continuous arterial spin labeling (CASL) perfusion images for precise grading of the severity in a murine model of endovascular perforation model of SAH.
Purpose
To investigate the feasibility of MRI-based assessment of clot distribution and CBF for grading the severity in a mouse endovascular perforation model of SAH.Methods
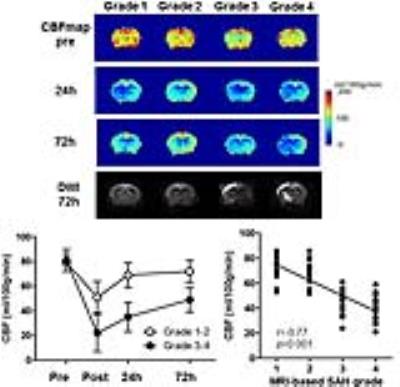
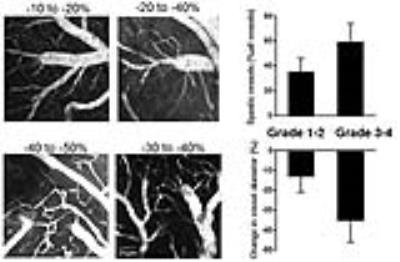
All experimental protocols in this study were approved by the Institutional Animal Care and Use Committee at the Research Institute. SAH was induced by endovascular perforation in 60 adult male C57BL/6 mice. CBF was estimated by 4.7 Tesla MRI of continuous arterial spin labeling (CASL) using a two-coil system with labeling neck coil and quadrature brain surface coil (Rapid Biomedical, Germany). CASL was acquired with a gradient echo sequence (TR/TE =8/4 ms, FA = 20º, FOV = 20 × 20 mm, matrix of 64 × 64, post labeling delay = 400 ms; number of excitations = 8; scan time: 1 min 25 sec). The CBF value was calculated as λ/T1b × (Mlabel – Mref)/(Mlabel + (2α -1)Mref), where Mlabel is the brain tissue signal acquired by brain surface coil 3 sec after RF irradiation at a labeling position for neck coil and Mref is the signal with RF irradiation of control position [2]. For neuro-behavioral assessment, modified Garcia neuroscore, adhesion removal test, and open field tests were recorded. MRI and neuro-behavioral performance were assessed immediately after SAH induction and at 24h and 72h after SAH (Figs. 1-3). Each parameter and their relationships with MRI-based grading score using 3-dimentional T2*-weighted image (long TR/TE= 20/10 ms, FA = 20°, FOV = 20 × 20 × 20 mm3, matrix size of 128 × 128 × 128; scan time: 5 min 29 sec) [3], CBF values, or new ischemic lesion detected by diffusion-weight image (DWI) (TR/TE = 2000/80 ms, FOV = 20 × 20 mm2, matrix size = 128 RO × 64 PE zero filled to 128 × 128 [b-value 1008.7; large delta 40 ms; small delta 10 ms]; slice interval 1.0 mm, thickness 0.8 mm [slice gap 0.2 mm]; measurement time: 2 min 18 sec) [4]. Cortical vasospasm visualized by two-photon laser microscopy (FV1000-2P; Olympus, Japan) were also analyzed for evaluation of delayed microvascular impairment between 48h and 72h. After visual verification of the major middle cerebral artery branch by an intravenous injection of sulforhodamine 101, volume of interest in the watershed area of the somatosensory cortex (avoid the cortical large vessels) was selected and the images were acquired up to a maximum depth of 400 μm from the cortical surface.Results
There were strong correlations between the CBF value at 24 h after SAH induction and T2*WI-based SAH grading (r=0.75; p<0.001) (Fig. 1), incidence of cortical microvasospasm on two photon microscopty (r=0.68; p<0.001) (Fig. 2), DWI-evidenced new ischemic lesion (r=0.61; p<0.001) (Fig. 1), or functional outcome score (r=0.81; p<0.001) (Fig. 3) measured 72h later after the SAH. Activity as measured by mean daily velocity (r=0.54; p=0.008) or position rotation ratio (r=0.47; p=0.03) at 24h had moderate correlation with the CBF value (Fig.4).Conclusions
Non-invasive MRI-based grading that includes both CBF and neurobehavioral assessment at 24 hours enables accurate evaluation of murine SAH severity based on the prevalence of microcirculatory impairment, DCI and functional outcome.Acknowledgements
This study was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (15K10966), Life Science Foundation of Japan, and Cooperative Research Project Program of Joint Usage/Research Center at the IDAC, Tohoku University.References
1. Mutoh T, Kazumata K, Terasaka S, et al. Early intensive versus minimally invasive approach to postoperative hemodynamic management after subarachnoid hemorrhage. Stroke 2014; 45:1280-1284.
2. Nakamura K, Wright D, Kondoh, et al. Quantitative accuracy of delayed hyperperfusion in mri of transient ischemia in rats. Conf Proc IEEE Eng Med Biol Soc 2008; 2008:839-842.
3. Mutoh T, Mutoh T, Sasaki K, et al. Value of Three-dimensional maximum intensity projection display to assist in magnetic resonance imaging (MRI)-based grading in a mouse model of subarachnoid hemorrhage. Med Sci Monit 2016; 22:2050-2055.
4. Mutoh T, Mutoh T, Sasaki K, et al. Isoflurane postconditioning with cardiac support promotes recovery from early brain injury in mice after severe subarachnoid hemorrhage. Life Sci 2016; 153:35-40.
Figures