2442
Excitatory Repetitive Transcranial Magnetic Stimulation (rTMS) Induces Contralesional Cortex-Cerebellar Pathways to Facilitate Motor Recovery after Acute Ischemic Stroke1Department of Radiology, Peking Union Medical College Hospital, Peking Union Medical College & Chinese Academy of Medical Sciences, Beijing, People's Republic of China, 2State Key Laboratory of Brain and Cognitive Science, Beijing MR Center for Brain Research, Institute of Biophysics, Chinese Academy of Sciences, Beijing, People's Republic of China, 3Department of Interventional Radiology, China Meitan General Hospital, Beijing, People's Republic of China, 4Department of Radiology, Department of Radiology, School of Medicine, University of Pennsylvania, Philadelphia, PA, United States, 5Department of Neurology, Peking Union Medical College Hospital, Peking Union Medical College & Chinese Academy of Medical Sciences, Beijing, People's Republic of China
Synopsis
In this study we used the Diffusion Tensor Imaging method to assess motor pathway changes in acute ischemic stroke patients after high frequency repetitive transcranial magnetic stimulation (rTMS). We found that the excitatory rTMS applied to the ipsilesional primary motor cortex induced the contralesional cortex-cerebellar loop to facilitate motor recovery. The result is consistent with those of our former studies and gives us a clue to understand the therapeutic mechanism of rTMS for early stroke patients.
PURPOSE
Repetitive transcranial magnetic stimulation (rTMS) is proved to be an effective treatment for neurological and psychiatric disorders. However, its therapeutic mechanism is unclear. Our study aimed to investigate motor pathway changes after excitatory rTMS in acute ischemic stroke patients.METHODS
Twelve patients with unilateral cerebral subcortex lesion in the middle cerebral artery territory detected by diffusion weighted image (DWI) were recruited in our study. They were separated into 2 groups randomly. The treatment group was consisted of 6 patients and received a 10-day rTMS treatment applied to the ipsilesional primary motor area (M1) beginning at about 4 days after stroke onset. The stimulation involved 50 trains of 20 pulses each day over the ipsilesional M1 at a frequency of 5HZ, with the stimulus intensity set at 120% of the resting motor threshold of the unaffected extremity. The remaining 6 patients received sham rTMS. Diffusion tensor imaging (DTI) data were collected in every patient before and after the rTMS or sham rTMS. DTI was performed on a 3.0 Tesla Magnetic Resonance Imaging (MRI) System (MAGNETOM Skyra System, Siemens, Erlangen, Germany) by using a twenty-channel phased-array head coil. Processing of the DTI data was implemented using a pipeline toolbox, PANDA1.Voxel-based analysis was used to study the difference in fractional anisotropy (FA) between the two groups using the REST software2. Changes of brain regions were projected onto an anatomical template (Ch2.nii) originally found in MRIcro (http://www.cabiatl.com/mricro/).RESULTS
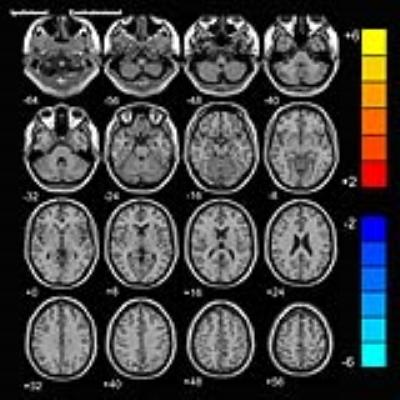
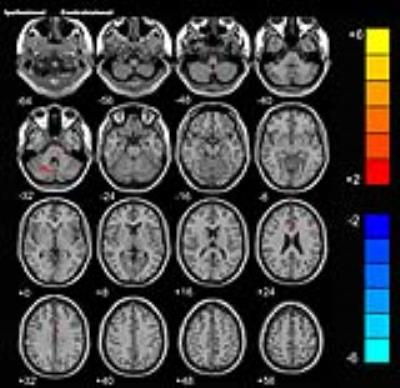
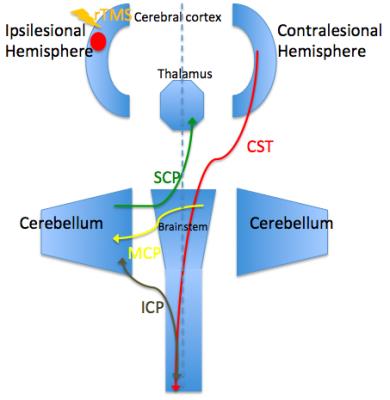
Before the rTMS, there is no significant difference in FA between the two groups. While after the treatment, the rTMS group showed increased FA in the contralesional corticospinal tract (CST), the pontine crossing tract, the middle cerebellar peduncle, the contralesional superior cerebellar peduncle, the contralesional medial lemniscus, and the ipsilesional inferior cerebellar peduncle. These fasciculi comprise the cortex-pontine-cerebellum-cortex loop. Increased FA was also found in the body of corpus callosum and the contralesional cingulum of the treatment group compared with the sham. The contralesional posterior thalamic radiation and the contralesional posterior corona radiata showed decreased FA after the rTMS.DISCUSSION
Brain reorganization after stroke is a research hotspot. However, it is challenging to delineate this functional reorganization in vivo through imaging techniques. DTI is a non-invasive approach to display and analysis white matter.3, 4 We used DTI in our study and found increased FA of the contralesional CST, the pontine crossing tract and the cerebellar peduncles in the treatment group. The result was very enlightening and worth great attention. Dr. Zhang and Dr. Mori identified decreased FA in the ipsilesional CST and contralesional cerebellar fibers in stroke patients.5 Our study applied high frequency rTMS to stroke patients and found increased FA of the contralesional cortex-cerebellar loop. Hemispheric changes in the above studies are opposite and the main difference between the two studies was whether rTMS was carried out. From this perspective, we can speculate that the rTMS induced reorganization of contralesional cortex-cerebellar fibers to compensate abnormalities of the ipsilesional brain areas, thus facilitating motor recovery. In our previous research, we used resting-state functional MRI and observed motor recovery along with increased functional connectivity of ipsilesional M1 with homologous motor areas in the contralesional hemisphere after rTMS.6 Then it is essential to figure out whether the fibers connecting these activated areas are changed. In this study, we also found increased FA of the corpus callosum and the cingulum. Reorganization of these commissural fibers indicated the strengthening communication of the two hemispheres. Through this communication, the contralesional motor related areas could compensate better for the function of the ipsilesional one. This answered the question mentioned above that motor fibers between the activated areas in our former study were changed after the rTMS. The rTMS facilitates brain reorganization of the contralesional hemisphere not only by activating the function of motor related regions but also strengthening the fibers connecting these regions. However, due to our small sample size, we only provide preliminary evidence in support of the therapeutic mechanism of rTMS for stroke patients. Further investigation is warranted to replicate these results.CONCLUSION
We found increased integrity of contralesional cortex-cerebellar loop after the excitatory rTMS by using the DTI method. This is consistent with our former studies and gives us a clue to understand the mechanism of rTMS in motor recovery of early stroke patients.Acknowledgements
This work was supported by funds from the National Natural Science Foundation of China (81271545), The Scientific Research Foundation for the Returned Overseas Chinese Scholars, The Science and Technology Foundation for the Selected Returned Overseas Chinese Scholars, and The Youth Foundation of Peking Union Medical College Hospital (2010104).References
1. Cui Z, Zhong S, Xu P et al. PANDA: a pipeline toolbox for analyzing brain diffusion images. Frontiers in Human Neuroscience. 2013 Feb 21;7:42. doi: 10.3389/fnhum.2013.00042. eCollection 2013.
2. Song XW, Dong ZY, Long XY et al. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011; 6: e25031.
3. Peter J. Basser JM, LeBihan D. MR Diffusion Tensor Spectroscopy and Imaging. Biophysical Journal. 1994; 66: 259-267.
4. Xue R, Sawada M, Goto S, et al. Rapid Three-Dimensional Diffusion MRI Facilitates the Study of Acute Stroke in Mice. Magnetic Resonance in Medicine. 2001; 46: 183-188.
5. Zhang W, Li X, Zhang J et al. Landmark-referenced voxel-based analysis of diffusion tensor images of the brainstem white matter tracts: application in patients with middle cerebral artery stroke. Neuroimage 2009; 44: 906-913.
6. Li J, Zhang XW, Zuo ZT et al. Cerebral Functional Reorganization in Ischemic Stroke after Repetitive Transcranial Magnetic Stimulation: An fMRI Study. CNS Neuroscience & Therapeutics. Version of Record online: 15 JUL 2016| DOI: 10.1111/cns.12593.
Figures