2436
A SEMI-AUTOMATIC METHOD TO SEGMENT MULTIPLE SCLEROSIS LESIONS ON FLAIR MAGNETIC RESONANCE IMAGES1Neuroimaging Research Unit, San Raffaele Scientific Institute, Vita-Salute San Raffaele University, Milan, Italy, 2Department of Neurology, San Raffaele Scientific Institute, Vita-Salute San Raffaele University, Milan, Italy, 3Department of Neuroradiology, San Raffaele Scientific Institute, Vita-Salute San Raffaele University, Milan, Italy
Synopsis
Aim of the study was to adapt and evaluate on FLAIR images a recently developed semi-automatic method for segmentation of hyperintense multiple sclerosis (MS) lesions on dual-echo (DE) PD/T2-weighted MRI. FLAIR MRI scans were obtained from 17 clinically isolated syndromes patients on a 1.5T scanner. The method was based on a region growing approach initialized by manual identification of lesions. The stop condition was formulated combining intensity and edge detection constraints. High similarity with the manual segmentation (the gold standard) was found, as well as a low misclassification of lesion voxels. Operator time required for lesion segmentation was importantly reduced.
Purpose
The analysis of disease burden using MR images from patients with multiple sclerosis (MS), both for research and clinical trials, requires the quantification of the volume of hyperintense lesions on a T2-weighted MRI sequence.1 During the last years, several automatic methods for MS lesion segmentation have been proposed.2 The majority of these techniques have been optimized and validated on FLAIR MR sequences, that benefit from CSF signal suppression and better contrast between focal lesions and the surrounding tissue, compared to dual-echo (DE) PD/T2-weighted MRI scans. However, one of the open issues for these methods remains the high rate of false positive and false negative lesions identified.3,4 Therefore, manual segmentation is still the gold standard. Aim of this study is to adapt and validate on FLAIR MR images a semi-automatic method we recently developed for MS lesion segmentation on DE MRI.5,6Methods
FLAIR MRI scans (TR/TE = 8000/90 ms; flip angle = 90°) were obtained on a 1.5T Philips Achieva scanner from 17 patients with clinically isolated syndrome (CIS) suggestive of MS (mean lesion load=2.5 ±2.3 ml). Adapting the method to the FLAIR sequences, the intensity standardization and the first training to find the optimal threshold for the stop condition was avoided. This is due to the fact that the intensity threshold was found estimating the distribution of the seed points and normalizing it we could find the weight for the maximum gradient for the lesion segmentation. The core of the algorithm remained the pixel-based region growing segmentation method: this approach examines neighbouring pixels of initial "seed points" and determines whether the pixel neighbours should be added to the region according to similarity constraints. Thus, starting from the seed point (manually identified by an expert physician), the expansion of the segmented region continued to the adjacent pixels until for all neighbouring pixels the stop condition was reached. This condition was a combination of two constraints. The first constraint examined whether the intensity of the new pixel was smaller than a computed threshold (Th_i):
$$Th_i = Iseed_i - w_i * G_i$$
Iseed_i was the image intensity of the i-th seed point.
$$G_i = 0.25 * Iseed_i$$
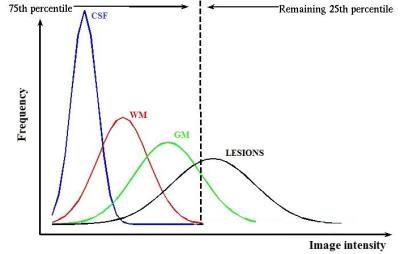
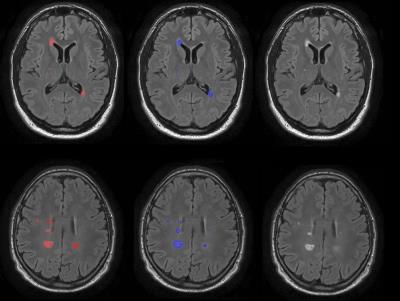
G_i and w_i were respectively the intensity gradient and the weight coefficient associated to the i-th seed point. The intensity gradient (G) was expressed considering that lesions are outliers on FLAIR histogram. Thus, defining the 75th percentile of the histogram as cut-off for the outliers (Figure 1), a new pixel cannot be segmented as lesion if its intensity is smaller than the 25% of the maximum intensity (i.e., the seed point intensity). Since the intensity of the seed point not always represents the maximum intensity on the FLAIR histogram, we introduced a weight coefficient (w) into the threshold computation, to correct for lesions heterogeneity. These coefficients were computed normalizing the distribution of all seed points of the image, to which they are associated. The second constraint for the stop condition examined whether a lesion edge was reached based on the high-pass filtering of the FLAIR image. The algorithm was implemented in Matlab®. Manual segmentations by an expert operator were used as the gold standard (Figure 2). The metrics evaluated were Dice Similarity Coefficient (DSC), Root Mean Squared Error (RMSE) of lesion load, True Positive Fraction (TPF), False Positive Fraction (FPF), and False Negative Fraction (FNF) for each patient.
Results
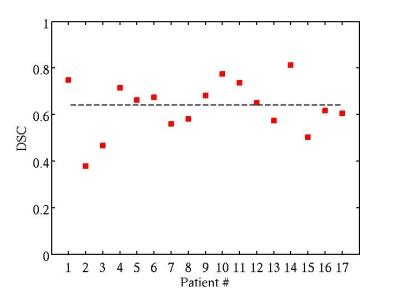
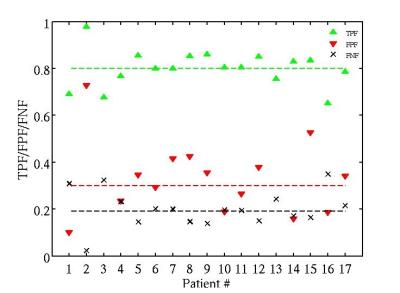
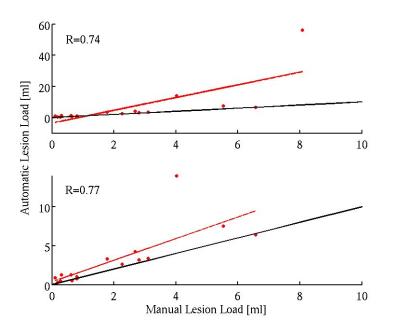
The validation measures averaged over all patients were obtained: DSC = 64% (Figure 3); TPF = 0.8; FPF = 0.32; FNF = 0.19 (Figure 4); RMSE = 0.65 ml (Figure 5).Discussion
The method showed high agreement with the gold standard, with high values of similarity coefficients. FPF and FNF values indicated low misclassification of lesion voxels and low measurement error. Moreover, the operator time required to extract lesion volumes was importantly reduced. The method did not require training on manual segmentation, making it applicable for research and clinical trials.Conclusions
The introduction of an automatic method for lesion segmentation in MS is desirable. However, the high rate of false positive and false negative lesions identified by those techniques prevented their spread. The method proposed maintained the manual identification of lesions by an expert physician to avoid individuation of false positive and false negative lesions, while it allowed the acceleration of the lesion contouring process, that is the real time consuming task, and it performed very similarly to the gold standard.Acknowledgements
Partially supported by Fondazione Italiana Sclerosi Multiple (FISM2013/S/1).References
1. Filippi M., Rocca M.A., De stefano N., et al. Magnetic resonance techniques in multiple sclerosis: the present and the future. Arch Neurol, 2011; 68: 1514-20.
2. Garcìa-Lorenzo D., Francis S., Narayanan S., et al. Review of automatic segmentation methods of multiple sclerosis white matter lesions on conventional magnetic resonance imaging. Med Image Anal, 2013; 17:1-18.
3. Van Leemput K., Maes F., Vandermeulen D., et al. Automated segmentation of multiple sclerosis lesions by model outlier detection. IEEE Trans Med Imaging, 2001; 20:677-88.
4. Garcìa-Lorenzo D., Prima S., Arnold D.L., et al. Trimmed-Likelihood estimation for focal lesions and tissue segmentation in multi-sequence MRI for multiple sclerosis. IEEE Trans Med Imaging, 2011; 30: 1455-67.
5. Storelli L., Pagani E., Rocca M.A., et al. A semi-automatic method for segmentation of multiple sclerosis lesions on dual-echo magnetic resonance images. Lect Notes Comput Sci, 2016; 9556:80-90.
6. Storelli L., Pagani E., Rocca M.A., et al. A semiautomatic method for multiple sclerosis lesion segmentation on dual-echo MR imaging: application in a multicenter context. Am J Neuroradiol, 2016; 10.3174.
Figures