2433
Effects of coil combination algorithms on Quantitative Susceptibility Mapping1NMR Unit, Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany, 2Department of Psychiatry, Social Psychiatry and Psychotherapy, Hannover Medical School, Hannover, Germany, 3A. A. Martinos Center for Biomedical Imaging and Department of Radiology, Harvard Medical School, Boston, MA, United States
Synopsis
Quantitative Susceptibility Mapping is an imaging technique for obtaining information of the magnetic susceptibility from phase images. If the phase information is obtained from a coil composed of multiple receive elements (coil array), the resulting images need to be combined. This step is non-trivial, and several algorithms, with different performances, have been proposed. Here we compare two different coil combination algorithms: the adaptive combine method and a recently proposed extension of ESPIRiT. For each, we compared both single subject and group-averaged QSM maps, where we observed that the results are sensitive to the method used for coil combination.
Purpose
Quantitative Susceptibility Mapping (QSM) has emerged as novel imaging modality that allows for the estimation of the relative magnetic susceptibility of an object. The standard QSM methods rely on the phase evolution of the signal from a FLASH sequence to obtain the susceptibility maps through elaborated algorithms. Such phase images are often obtained through the combination of the signal detected separately by multiple receive coil elements (channels). Since each channel is subject to specific modulations of the complex signal, a simple linear combination of the signals can result in constructive or destructive interference due to the inconsistency of the phase offset across the different channels. To overcome this issue, several different coil combination algorithms have been proposed1,2. Some of them, including those implemented by a major MRI vendor3, while performing well for obtaining magnitude images, are known to degrade the phase images, especially in regions of relatively low SNR. Here, we investigate the potential impact of degraded phase images on QSM results.Methods
FLASH complex images of the full brain were obtained with:
- 3T Siemens MAGNETOM Verio operated under Syngo VB17;
- Tx: body coil, Rx: 32-channel Siemens head coil;
- 22 healthy volunteers (6 females, age: $$$19-57\;\mathrm{yr}$$$);
- $$$T_R=30\;\mathrm{ms}$$$, $$$T_E=17\;\mathrm{ms}$$$, $$$\alpha=13\mathrm{°}$$$, resolution: $$$0.8\;\mathrm{mm}$$$ (isotropic).
For each acquisition, we reconstructed the combined phase images from the single-channel complex data, using two different methods:
1. the adaptive (vendor’s standard) combination algorithm3;
2. a recently proposed ESPIRiT-based combination approach4,5 using data driven virtual conjugate coil estimation obtained from singular value decomposition (SVD)6
QSM images were obtained with an identical pipeline for both input phases. The QSM pipeline was based on the Superfast Dipole Inversion (SDI) approach7: a combination of the Sophisticated Harmonic Artifact Reduction in Phase data (SHARP) background field removal and Thresholded K-space Division (TKD) field-to-source inversion. The cerebrospinal fluid (CSF) of the lateral ventricles was used to fix the arbitrary reference of the QSM maps.
The results were analyzed both voxel-by-voxel and on a region-of-interest (ROI) basis. For both single-subject and the MNI-registered cohort of subjects, we computed the difference images, their mean, $$$\mu_D$$$, and standard deviation, $$$\sigma_D$$$, and 2D histograms. We also calculated the group average, standard deviations and Wilcoxon signed-rank non-parametric test for the different ROIs. The ROIs were obtained from the susceptibility maps with different approaches:
- basal ganglia: with the FSL-FIRST subcortical segmentation algorithm on optimized MP2RAGE-QSM hybrid contrast images8,9;
- brain stem (Red Nucleus; Substantia Nigra; Sub-thalamic Nucleus): using non-linear registration of the ATAG atlas to native space;
- brain cortex structures (insula and cingulum): with FreeSurfer.
Results
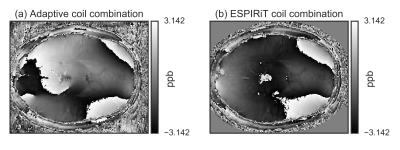
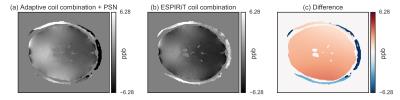
Fig.[1] shows an example of the improved performance of the ESPIRiT-based approach over the adaptive coil combination, which shows a severe phase artifact.
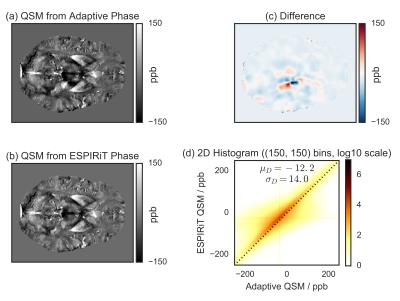
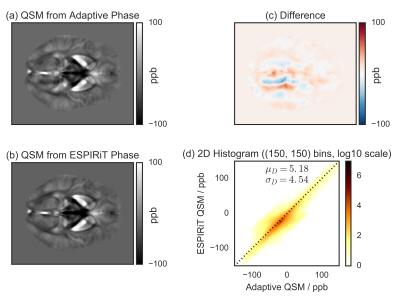
Single subject (Fig.[2]) and MNI-registered group average (Fig.[3]) QSM results are shown for the adaptive and the ESPIRiT phase images, along with their difference, and the 2D voxel-by-voxel histogram. The strongest difference occurs at the position of the phase artifact. Additionally, the difference image and the histogram indicate that the QSM results are also globally different.
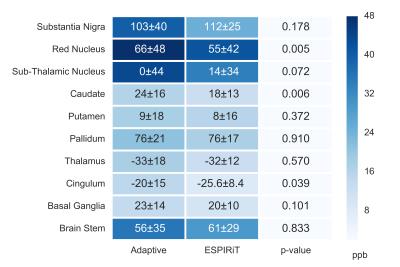
Fig.[4] reports the statistical analysis in selected ROIs for the QSM maps calculated from both default and corrected phase images.
Discussion
The most significant difference between the QSM images is the absence of significant left-to-right asymmetry in the ESPIRiT-based results. This originates from the absence of the artifact observed in the adaptive phase image.
The complex non-local relationship between the phase evolution and the magnetic susceptibility may be the reason for the global QSM degradation observed.
The finding is consistent across all subjects, as indicated by the group average results, because such artifacts are more likely to be localized in regions with poor SNR, whose position is greatly determined by the coil.
For the ESPIRiT coil combination, the ROI analysis indicates that the group variation (standard deviation) is reduced and the average value may be significantly (depending on the region) different from the results obtained from the standard phase. This is especially relevant for group studies were accurate results are crucial10.
Finally, note that the tested adaptive method (as implemented in our study) was improved in newer software versions11, where now a short reference scan acquired with the body coil can be used for calibration, thus obtaining accurate phase estimations (see Fig.[5]).
Conclusion
This study indicates that adaptive coil reconstruction may be unsuitable for reliable QSM results, and improved algorithms are required for group studies, even when considering ROIs that do not seem degraded by visual inspection of either the input phase or the QSM results.Acknowledgements
We would like to thank the following people: Andreas Schäfer and Simon Robinson for helpful discussions and for kindly sharing source code; Toralf Mildner and Tobias Leutritz for additional support with data collection. Funded by: EU through the 'HiMR' Marie Curie ITN (FP7-PEOPLE-2012-ITN-316716), the Max Planck Society through IMPRS NeuroCom, and the Helmholtz Alliance 'ICEMED'.References
1. Robinson, S.D., Dymerska, B., Bogner, W., Barth, M., Zaric, O., Goluch, S., Grabner, G., Deligianni, X., Bieri, O., Trattnig, S., 2015. Combining phase images from array coils using a short echo time reference scan (COMPOSER). Magn. Reson. Med. doi:10.1002/mrm.26093
2. Robinson, S., Grabner, G., Witoszynskyj, S., Trattnig, S., 2011. Combining phase images from multi-channel RF coils using 3D phase offset maps derived from a dual-echo scan. Magn. Reson. Med. 65, 1638–1648. doi:10.1002/mrm.22753
3. Prock, T., Collins, D.J., Dzik-Jurasz, A.S.K., Leach, M.O., 2002. An algorithm for the optimum combination of data from arbitrary magnetic resonance phased array probes. Phys. Med. Biol. 47, N39. doi:10.1088/0031-9155/47/2/402
4. Uecker, M., Lai, P., Murphy, M.J., Virtue, P., Elad, M., Pauly, J.M., Vasanawala, S.S., Lustig, M., 2014. ESPIRiT—an eigenvalue approach to autocalibrating parallel MRI: Where SENSE meets GRAPPA. Magn. Reson. Med. 71, 990–1001. doi:10.1002/mrm.24751
5. Uecker, M., Lustig, M., 2016. Estimating absolute-phase maps using ESPIRiT and virtual conjugate coils. Magn. Reson. Med. doi:10.1002/mrm.26191
6. Bilgic, B., Polimeni, J.R., Wald, L.L., Setsompop, K., 2015. Automated tissue phase and QSM estimation from multichannel data, in: #2849. Presented at the ISMRM 23rd Annual Meeting & Exhibition, Toronto, Canada.
7. Schweser, F., Deistung, A., Sommer, K., Reichenbach, J.R., 2013. Toward online reconstruction of quantitative susceptibility maps: Superfast dipole inversion. Magn Reson Med 69, 1581–1593. doi:10.1002/mrm.24405
8. Schweser, F., Feng, X., Mach Batlle, R., Güllmar, D., Deistung, A., Dwyer, M.G., Zivadinov, R., Reichenbach, J.R., 2015. Improved deep gray matter segmentation using anatomical information from quantitative susceptibility maps, in: #1787. Presented at the ISMRM 22nd Annual Meeting & Exhibition, Milan, Italy.
9. Feng, X., Deistung, A., Schweser, F., Güllmar, D., Reichenbach, J.R., 2015. Investigation of Brain Segmentation with FIRST by Using Different Hybrid Contrasts and Registrations, in: #3497. Presented at the ISMRM 23rd Annual Meeting & Exhibition, Toronto, Canada.
10. Kanaan, A.S., Schäfer, A., Metere, R., Bilgic, B., Müller-Vahl, K.R., Möller, H.E., 2016. Quantitative susceptibility mapping in Gilles de la Tourette syndrome. Presented at the 9th Annual Meeting of the European Society for the Study of Tourette Syndrome (ESSTS), Warsaw, Poland.
11. Jellúš, V., Kannengiesser, S.A.R., 2014. Adaptive Coil Combination Using a Body Coil Scan as Phase Reference, in: #4406. Presented at the ISMRM 22nd Annual Meeting & Exhibition, Milan, Italy.
Figures