2429
Distinct and common gray matter volume and cortical thickness abnormalities between non-comorbid medication-naive patients with major depressive disorder and social anxiety disorder1Department of Radiology,West China Hospital of Sichuan University, Huaxi MR Research Center (HMRRC), Chengdu, People's Republic of China
Synopsis
An overlap of diagnosis frequently occurs between major depression disorder (MDD) and social anxiety disorder (SAD) and few studies directly compare neuroanatomical abnormalities in the two disorders. Pure MDD patients (n = 37), pure SAD patients (n = 24) and healthy controls (n = 41) underwent T1-weighted magnetic resonance imaging (MRI). Gray matter volume and cortical thickness were compared in the three groups. The main findings of this study were that (i) MDD and SAD patients shared common neural substrates in frontal-subcortical circuits; and (ii) MDD patients manifested more widespread brain structure alterations than SAD patients.
Purpose
Major depressive disorder and social anxiety disorder has seen a frequent overlap of diagnosis between them and till date only few studies have directly compared the neuroanatomic abnormalities between these two disorders. The aim of this study was to access the alterations of gray matter volume and cortical thickness between MDD and SAD patients.Methods
Participants were diagnosed with pure major depressive disorders (MDD) (n = 37, M/F=26/15, mean age 27.1±7.2 years), pure social anxiety disorder (SAD) (n = 24, M/F=25/12, mean age 26.7±7.1 years), and healthy controls (healthy controls; n = 41, M/F=15/9, mean age 23.8±4.5 years), who were free from Axis-I disorder. In a cross-sectional design, high-resolution T1-weighted images using a volumetric 3D Spoiled Gradient Recall sequence were acquired from all subjects. Gray matter volume (GMV) and cortical thickness were compared between all the three groups. The GMV was calculated using optimized voxel-based morphometry (VBM), following diffeomorphic anatomical registration through exponentiated lie algebra (DARTEL) 1 using Statistical Parametric Mapping software (SPM8, http://www.fil.ion.ucl.ac.uk/spm). Whole-brain voxelwise analysis tested a main effect of diagnosis (MDD, SAD and HC) using a factorial design in SPM8. Voxel-wise ANOVA and post-hoc pairwise comparisons (MDD vs. HC, SAD vs. HC, and MDD vs. SAD) were performed with the threshold set at p < 0.05 corrected by family-wise error (FWE). Cortical reconstruction and estimation of cortical thickness were performed with the FreeSurfer package 2 (version 5.1.0, http://surfer.nmr.mgh.harvard.edu/). Moreover, GMV maps were analyzed in the context of the general linear model. Regional cortical thickness comparisons were performed using the SPSS ANOVA models followed by post hoc pairwise comparisons. The results were further corrected for multiple comparisons using FWE approach.Results
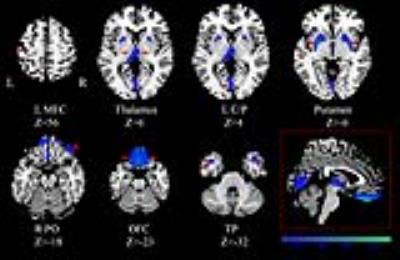
Volumetric analysis demonstrated significant abnormalities in cortical and subcortical gray matter, notably including GMV reductions in the bilateral orbital frontal cortex (OFC), putamen and thalamus in both patient groups when compared to HC. Besides, only the MDD patients showed decreased GMV in the left middle frontal cortex (MFC), bilateral cuneus/ pericalcarine and increased GMV in the bilateral temporal pole (TP) than HC. However, no statistically significant volumetric difference was observed between MDD and SAD groups in GMV. Surface-based analysis demonstrated cortical abnormalities in both groups of patients involving frontal, temporal, parietal and occipital lobe regions. Compared with SAD patients, MDD patients showed cortical thinning of the left pericalcarine and cortical thickening of the left fusiform. Both patient groups showed increased cortical thickness in the bilateral anterior cingulate cortex, posterior cingulate cortex, inferior temporal cortex, middle temporal cortex, TP and left superior frontal cortex (SFC), caudal MFC; decreased cortical thickness in the left rostral MFC when compared to HC. In addition, only MDD patients showed reduced thicknesses in bilateral pericalcarine, bilateral fusiform, right caudal MFC, right lateral OFC, and right SFC relative to HC.Discussion & Conclusion
The main finding of this study was that MDD and SAD patients shared common neural substrates and MDD patients manifested more widespread brain structure alterations than SAD patients. In both groups, GMV was found to be decreased in bilateral OFC, putamen and thalamus which is consistent with previous studies showing structure abnormalities in frontal-subcortical circuits. 3,4 However, greater cortical thickness was observed in variety of brain regions mainly involving frontal, temporal, parietal and occipital lobes. One of the regions of the brain regions showing a significant cortical change than GMV change might be due to the sensitivity of cortical based methods in detecting structural difference than volume based methods.5 The involvement of different regions of GMV reduction and increase in cortical thickness may reflect a compensatory mechanism to cope with less efficient of frontal-subcortical circuits in major depression and social anxiety. On comparison with HC, MDD patients manifested more widespread brain structure alterations than SAD patients. MDD patients showed cortical thinning in left pericalcarine and thickening in left fusiform than SAD patients. Because high trait anxiety is considered as a risk factor for major depression,6,7 there is reason to assume that common neural components represent general risk factors for psychopathology. Due to different factors present at illness onset, these neural components might lead to distinct forms of psychopathology with unique neural signatures in MDD and SAD8. Taken together, these findings suggest that common structure changes involved in mood and emotional regulation may be shared in major depression and social anxiety. In addition, MDD patients have shown more widespread structure alterations than SAD patients.Acknowledgements
This study was supported by the National Natural Science Foundation (Grant Nos. 81000605, 81220108013 and 0040205401A59). Q. Gong also acknowledges his Visiting Professor appointment in the Department of Psychiatry at the Yale School of Medicine, Yale University, USA.References
1. Ashburner, J. (2007). A fast diffeomorphic image registration algorithm. Neuroimage, 38(1), 95-113.
2. Fischl, B., & Dale, A. M. (2000). Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A, 97(20), 11050-11055. doi: 10.1073/pnas.200033797
3. Talati, A., Pantazatos, S. P., Schneier, F. R., Weissman, M. M., & Hirsch, J. (2013). Gray matter abnormalities in social anxiety disorder: primary, replication, and specificity studies. Biol Psychiatry, 73(1), 75-84.
4. Nugent, A. C., Davis, R. M., Zarate, C. A., Jr., & Drevets, W. C. (2013). Reduced thalamic volumes in major depressive disorder. Psychiatry Res, 213(3), 179-185.
5. Hutton, C., Draganski, B., Ashburner, J., & Weiskopf, N. (2009). A comparison between voxel-based cortical thickness and voxel-based morphometry in normal aging. Neuroimage, 48(2), 371-380.
6. Sandi, C., & Richter-Levin, G. (2009). From high anxiety trait to depression: a neurocognitive hypothesis. Trends Neurosci, 32(6), 312-320.
7. Beesdo, K., Bittner, A., Pine, D. S., Stein, M. B., Hofler, M., Lieb, R., & Wittchen, H. U. (2007). Incidence of social anxiety disorder and the consistent risk for secondary depression in the first three decades of life. Arch Gen Psychiatry, 64(8), 903-912.
8. Hamilton, J. P., Chen, M. C., Waugh, C. E., Joormann, J., & Gotlib, I. H. (2015). Distinctive and common neural underpinnings of major depression, social anxiety, and their comorbidity. Soc Cogn Affect Neurosci, 10(4), 552-560.