2420
The Nulling Signal Produced by Inversion Recovery for Gray Matter is Related to Neuronal Density in the Thalamic Subnuclei: A 7T MRI Study1Neuroscience Research Institute, Gachon university, Incheon, Korea, Republic of, 2Department of Biomedical Engineering, Gachon University, 3Department of Neurology, Gachon University Gil Medical Center, Gachon University, 4Department of Radiological Sciences, Gachon University
Synopsis
The thalamus acts as a gateway to higher brain centers that are involved in cognition, sleep, arousal, as well as sensory and motor information 1–5. Additionally, changes in the thalamic subnuclei (TS) have been implicated in a number of neuropsychiatric disorders. In a postmortem study, the neuronal number as well as the total volume of the thalamus were significantly decreased in schizophrenic subjects or elevated in major depression subjects, especially in the medial dorsal nucleus 6,7. As a result, the ability to estimate neuronal density in the thalamic subnuclei (TS) using in-vivo imaging is highly desired.
SYNOPSIS
The thalamus acts as a gateway to higher brain centers that are involved in cognition, sleep, arousal, as well as sensory and motor information 1–5. Additionally, changes in the thalamic subnuclei (TS) have been implicated in a number of neuropsychiatric disorders. In a postmortem study, the neuronal number as well as the total volume of the thalamus were significantly decreased in schizophrenic subjects or elevated in major depression subjects, especially in the medial dorsal nucleus 6,7. As a result, the ability to estimate neuronal density in the thalamic subnuclei (TS) using in-vivo imaging is highly desired.PURPOSE
To investigate whether the amount of signal nulling by the IR sequence tuned at suppressing gray matter is proportional to the amount of neuronal cell density in the TS using 7T MRI.METHODS
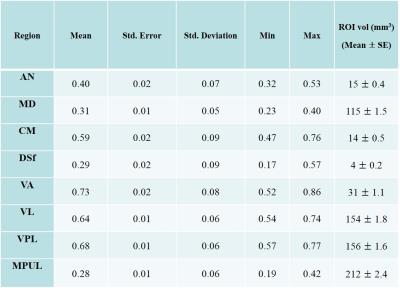
Ten healthy volunteers (female/male = 5/5; mean age = 25 years old; age range = 19 - 30) were recruited for the study. A 3-D MPRAGE sequence was obtained with inversion recovery time (T1) of 1000 ms. We defined eight volume-of-interest (VOI) pairs in the bilateral thalamus: the anterior nucleus (AN), medial dorsal nucleus (MD), dorsal superficial nucleus (DSf), ventral anterior nucleus (VA), ventral lateral nucleus (VL), ventral posterior lateral (VPL), centromedial nucleus (CM) and the medial pulvinar nucleus (MPUL). To make VOIs comparable with postmortem study, the VOIs were clustered into four groups based on previously published cytoarchitectural clusters 13, which consisted of the anterior (AN), medial (MD), lateral (VA, VL, VPL), and posterior nucleus (MPUL). The signal reduction ratio (SRR) was calculated by dividing the mean signal intensity of each group by the mean signal intensity of the internal capsule (reference VOIs) which is composed of pure white matter. Pearson's correlation coefficients (r) were calculated between the SRR and neuronal cell density of published cytoarchitectural data.RESULTS
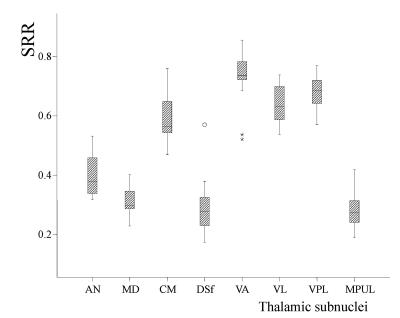
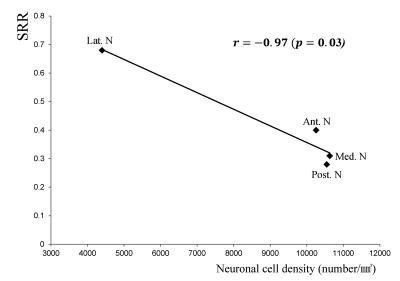
The calculated SRR and drawing VOI size of each TS are shown in Fig. 1. Based on the SRR values, the TS was separated into two groups consisting of the AN, MD, DSf and MPUL, which had relatively lower signal intensities, and the CM, VA, VL and VPL, which showed relatively higher SRR values (Fig. 2). The correlation coefficient between the SRR and neuronal density of the grouped TS was -0.97 (p = 0.03) (Fig. 3)DISCUSSION
Traditionally, attempts at delineating the TS with IR sequences have focused on T1 contrast enhancement, which can be achieved by nulling either white matter or gray matter using different inversion times 8–11. We hypothesized that fiber size and density differences among the TS would result in differences in contrast throughout the thalamus 12. Therefore, it is plausible that the degree of signal reduction nulled by an IR sequence tuned at gray matter could be proportional to the neuronal concentration. Our results showed a strong, negative correlation between total neuron density (macro plus micro neuronal cell density) and the SRR (r = -0.97).CONCLUSION
The study showed a strong correlation between the degree of IR signal attenuation and neuronal cell density, which suggests that the neuronal cell density of the TS can be estimated by applying IR sequences in living human brains.Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (No.2015R1C1A1A02036462 & 2015M3D5A1065683).References
1. Sherman SM. Thalamic relays and cortical functioning. Prog. Brain Res. 2005;149:107–126.
2. Guillery RW. Anatomical evidence concerning the role of the thalamus in corticocortical communication: a brief review. J. Anat. 1995;187 (Pt 3):583–592.
3. McCormick DA, Bal T. Sleep and arousal: thalamocortical mechanisms. Annu. Rev. Neurosci. 1997;20:185–215.
4. Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science 1993;262(5134):679–685.
5. Fama R, Sullivan EV. Thalamic structures and associated cognitive functions: Relations with age and aging. Neurosci. Biobehav. Rev. 2015;54:29–37.
6. Byne W, Fernandes J, Haroutunian V, et al. Reduction of right medial pulvinar volume and neuron number in schizophrenia. Schizophr. Res. 2007;90(1-3):71–75.
7. Pakkenberg B. Pronounced reduction of total neuron number in mediodorsal thalamic nucleus and nucleus accumbens in schizophrenics. Arch. Gen. Psychiatry 1990;47(11):1023–1028.
8. Cho Z-H, Son Y-D, Kim H-K, et al. Observation of glucose metabolism in the thalamic nuclei by fusion PET/MRI. J. Nucl. Med. 2011;52(3):401–404.
9. Magnotta VA, Gold S, Andreasen NC, et al. Visualization of subthalamic nuclei with cortex attenuated inversion recovery MR imaging. NeuroImage 2000;11(4):341–346.
10. Tourdias T, Saranathan M, Levesque IR, et al. Visualization of intra-thalamic nuclei with optimized white-matter-nulled MPRAGE at 7T. NeuroImage 2014;84(1):534–545.
11. Vassal F, Coste J, Derost P, Mendes V, et al. Direct stereotactic targeting of the ventrointermediate nucleus of the thalamus based on anatomic 1.5-T MRI mapping with a white matter attenuated inversion recovery (WAIR) sequence. Brain Stimulat. 2012;5(4):625–633.
12. Deoni SCL, Josseau MJC, Rutt BK, et al. Visualization of thalamic nuclei on high resolution, multi-averaged T1 and T2 maps acquired at 1.5 T. Hum. Brain Mapp. 2005;25(3):353–359.
13. Dom R., Neostriatal and thalamic interneurons. PhD. Dissertation; 1976:37-54.
Figures