2410
Harmonization for fractional anisotropy measurement at 3T in healthy subjects1Department of Diagnostic Radiology, the First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, People's Republic of China, 2Department of Biomedical Engineering, School of Life Science and Technology, Xi’an Jiaotong University
Synopsis
In current clinical practice, the absence of standard protocol of diffusion tensor imaging (DTI) challenges the consistency of DTI data, and thus affects the accuracy of data group analysis. This study aims to assess the inconsistency of DTI-derived fractional anisotropy (FA) based on the different clinical protocols and further make them into harmonization. Results suggest the effects of slice thickness and b-value of FA are anatomy dependent, which is more stable in white matter with packed and orientation consistent fibers. Besides, a developed polynomial fitting model was used to make FA harmonization among different DTI protocols.
Introduction
In current clinical practice, the absence of standard protocol of diffusion tensor imaging (DTI) challenges the consistency of DTI data. Especially, the selection of scanning parameters, e.g. b-value would affect the values of DTI-derived fractional anisotropy (FA), and thus affects the accuracy of data group analysis.1 Targeting such issue, previous studies have analyzed the inter- and intra-site consistency of DTI data.2,3 However, harmonization for DTI data with varying scan parameters remains lacking. From this, this study aims to assess the inconsistency of FA based on the different clinical protocols and further make them into harmonization.Methods
Subjects Five healthy volunteers (female/male=2/3; age range, 21~23 years) participated in this study. MR Protocols All DTIs were performed using a 3.0T scanner (GE, Signa HDxt) with an 8-channel head coil. Single shot spin-echo echo-planar DTIs were acquired using 4 protocols different in b-value, slice thickness and numbers of diffusion gradient directions (NDGD) based on our clinical settings (Table 1). Other parameters were: TR/TE=13000ms/minimum, matrix=172×172, FOV=24 cm. Data and statistical analysis DTI data was processed with the aid of the FMRIB software library (FSL, www.fmrib.ox.au.ukt/fsl). In terms of the adult atlas (JHU-white matter temples), regions of interest (ROIs) were defined in splenium of corpus callosum (SCC), genus of corpus callosum (GCC), bilateral posterior limb of internal capsule (PLIC) and bilateral corona radiata (CR). Polynomial fitting was separately used to correlate the FA values in each ROI between protocols according to the single changed factor. Since the protocol 1 as the reference, FA values in other 3 protocols were corrected by the polynomial fitting model. The mean and relative different rate of FA was calculated before and after correction. All statistical analysis were performed by using SPSS 17.0 (SPSS, Chicago, IL, USA); p<0.05 was considered as statistically significant difference.Results
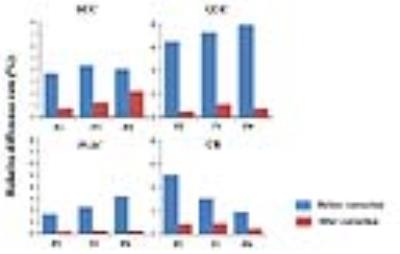
The various FA values were computed by DTI using different protocols within the SCC, GCC, PLIC and CR (Table 2). Significantly differences were found in SCC, GCC and CR (pSCC=0.006, pGCC<0.001, pCR=0.012) in terms of the changed slice thickness, only except for PLIC (pPLIC=0.333). When b-value elevated from 600s/mm2 to 1000s/mm2, the FA values of PLIC and CR were significantly different (pPLIC<0.001, pCR=0.012) but there was no difference in SCC and GCC (pSCC=0.354, pGCC=0.066).In addition, 5 NDGD changed showed no statistically difference in all ROIs. After the correction, mean FA values were very close (Fig.1) and the relative different rate in each ROIs was obviously decreased (Fig.2, Table 3).Discussion
This study indicated that the diversity of FA values among different protocols, and suggested the significance of harmonization before data analysis. ROI-based differences were found both in slice thickness and b-value changed, but not correlation with the NDGD in our protocol, which was consistent with previous studies.1,4,5 Thickened slice and higher b-value have the similar effects of decreasing FA. Compared with GCC and CR, SCC and PILC performed relatively good consistency, suggesting the density and directivity of fiber bundles may contribute to such differences. Furthermore, region-specific polynomial correction model was proposed to closely harmonize the FA across different protocols and obtained good consistency results with significantly reduction of relative different rate (less than 2.2%). Since there was no statistically difference between mean FA values after correction, this model owns the potential to be used in other DTI parameters in the future.Conclusion
The effects of slice thickness and b-value of FA are anatomy dependent, which is more stable in white matter with packed and orientation consistent fibers. The developed polynomial fitting model could be employed to harmonize the FA value among different DTI protocols.Acknowledgements
This work was supported by the National Key Research and Development Program of China (2016YFC0100300), National Natural Science Foundation of China (No.81171317, 81471631), and the 2011 New Century Excellent Talent Support Plan of the Ministry of Education, China (NCET-11-0438).References
1. Hui E S, Cheung M M, Chan K C, et al. B-value dependence of DTI quantitation and sensitivity in detecting neural tissue changes[J]. Neuroimage, 2010, 49(3): 2366-2374.
2. Pohl K M, Sullivan E V, Rohlfing T, et al. Harmonizing DTI measurements across scanners to examine the development of white matter microstructure in 803 adolescents of the NCANDA study[J]. NeuroImage, 2016, 130: 194-213.
3. Mirzaalian H, Ning L, Savadjiev P, et al. Inter-site and inter-scanner diffusion MRI data harmonization[J]. NeuroImage, 2016, 135: 311-323.
4. Bisdas S, Bohning D E, Bešenski N, et al. Reproducibility, interrater agreement, and age-related changes of fractional anisotropy measures at 3T in healthy subjects: effect of the applied b-value[J]. American Journal of Neuroradiology, 2008, 29(6): 1128-1133.
5. Ni H, Kavcic V, Zhu T, et al. Effects of number of diffusion gradient directions on derived diffusion tensor imaging indices in human brain[J]. American journal of neuroradiology, 2006, 27(8): 1776-1781.
Figures