2408
Carbon fiber electrodes for single-unit recording combined with artifact-free MRI1Department of Chemical Engineering, Stanford University, Stanford, CA, United States, 2Department of Neurology and Neurological Sciences, Stanford University, 3Departments of Bioengineering, Neurosurgery, Electrical Engineering, Stanford University, Stanford University
Synopsis
Combining unit recording and fMRI is a powerful method that allows for interrogation of the brain at the cellular and network level. However, implanted metal recording electrodes produce susceptibility artifacts that distort MR images. Here, we fabricate single carbon-fiber electrodes and implant them into rat hippocampus and use an agarose phantom to quantify its MR distortion. These carbon-fiber electrodes record from a single unit in CA1 and distort a significantly lower volume of voxels compared to metal electrodes. These results lay the groundwork for an electrode design that can be used for single-unit recording and fMRI in same subjects.
Purpose
Recent advances have utilized carbon-fiber (CF) bundles as probes to record field potentials.1,2,3 These have been shown to reduce susceptibility artifact, but their large diameters are not suited for unit recordings. Additionally, smaller diameter CF bundles suffer from high tip impedance. Therefore, there is a need for MRI compatible electrodes capable of high signal-to-noise ratio (SNR) unit recordings. Recently, conductive polymers such as PEDOT have been used to decrease the tip impedances of different types of probes, increasing their SNR.4,5,6 Here, we demonstrate that single CF electrodes with the conductive polymer PEDOT can be used to record from single units in the hippocampus and cause a negligible distortion on MR images. This demonstrates the applicability of these probes in studies that combine single-unit recordings and fMRI.Methods
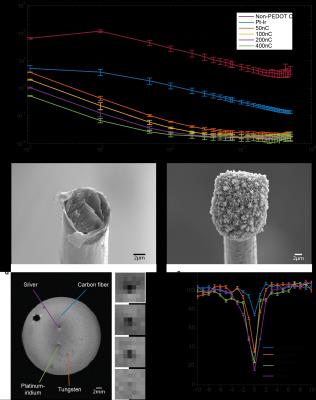
CF Electrode Fabrication: The tip of a single CF was polymerized in an electrodeposition process to make PEDOT:pTS electrodes. Impedance was measured from 1Hz to 5kHz and electrode tips were qualitatively analyzed using SEM.
MRI Phantom: Electrodes were embedded into a 0.6% Agarose phantom, and imaged using a 7T horizontal bore scanner and a multislice, multi-spinecho (MSME) sequence (TR=2120ms, TE=20, 40, 60, 80, 100, 120, 140, 180, 200ms, matrix=256x256, FOV=45x45mm2). One-dimensional signal intensity profiles were obtained by taking a row of voxels across each implantation site. Rows were chosen to contain the lowest pixel value in the center of the hypointense region and were averaged over ten echo times from the same slice.
Acute Recordings: All animals for acute recordings were handled in accordance with IUCAC. Surgery was conducted on a female Sprague-Dawley rat under 2-3% isofluorane.
Results
Electrode impedance spectra show decrease in impedance as a function of frequency across samples. PEDOT:pTS samples show decreasing impedance as a function of applied total charge during deposition and outperform unpolymerized and platinum-iridium (Pt-Ir) electrodes (Fig. 1a). While the differences in impedance are significant at lower frequencies, different coating thicknesses produce similar impedance values at higher frequencies, but in all cases outperform non-PEDOT coated electrodes. Furthermore, SEM imaging reveals noticeable morphological differences between the tips of non-PEDOT and PEDOT:pTS samples (Fig. 1b-c).
Single-fiber electrodes show a significantly reduced susceptibility artifact compared to silver (Ag), Pt-Ir, and tungsten (W) electrodes and is nearly undetectable in MR images (Fig. 1d). Furthermore, the 1D intensity profile show that even at the center of the hypointense region, pixel values in the single-fiber implantation site are no less than 70% of the local signal.
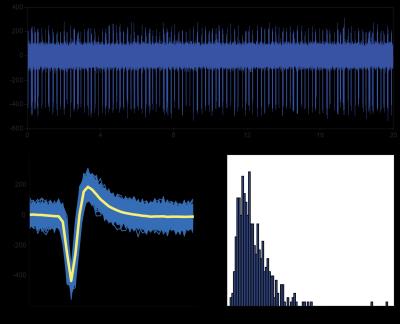
Raw traces show spiking events with amplitudes that are discernable from background noise (Fig. 2a). In particular, the overlay of detected spikes shows robust and consistent spike morphology (Fig. 2b), and the interspike interval histogram reveals spike onsets are Poisson distributed (Fig. 2c).
Discussion
We have highlighted the capability of single-fiber electrodes for in vivo unit recording in the rat hippocampus and demonstrate their potential for negligible distortion on MR images. Furthermore, single-fiber CF probes produce smaller susceptibility artifacts compared to other electrodes due to its low magnetic susceptibility and its small diameter (~10µm).
We have previously shown that CF bundle electrodes can record field potentials without causing significant artifacts in MR images.1 In comparison, the single-fiber electrodes show marked increase in performance over CF bundle and other metal electrodes, as they are capable of unit and field potential recordings and, as the data shown here demonstrates, they are small enough to be nearly invisible on spin-echo MR images.
Conclusion
Our work establishes that single CF electrodes can record from units and produce low susceptibility artifact in MR images, providing a tool that can be used to combine multi-site unit recording and low-distortion fMRI in parallel.Acknowledgements
This work was supported through funding from the NIH/NIBIB R00 Award (4R00EB008738), Okawa Foundation Research Grant Award, NIH Director’s New Innovator Award (1DP2OD007265), NSF CAREER Award (1056008), Alfred P. Sloan Foundation Research Fellowship, NIH/NINDS R01NS087159, NIH/NINDS R01NS091461, and NIH/NIA RF1AG047666.References
1. Duffy BA, Choy MK, Chuapoco MR, et al. MRI compatible optrodes for simultaneous LFP and optogenetic fMRI investigation of seizure-like afterdischarges. NeuroImage. 2016;123:173-184
2. Dunn JF, Tuor UI, Kmech J, et al. Functional brain mapping at 9.4T using a new MRI-compatible electrode chronically implanted in rats. Magn Reson Med. 2009;61(1):222-228
3. Jupp B, Williams JP, Tesiram YA, et al. MRI compatible electrodes for the induction of amygdala kindling in rats. J Neurosci Methods. 2006;155(1):72-76
4. Kozai TD, Langhals NB, Patel PR, et al. Ultrasmall implantable composite microelectrodes with bioactive surfaces for chronic neural interfaces. Nat Mater. 2012;11(12):1065-1073
5. Patel PR, Na K, Zhang H, et al. Insertion of linear 8.4 μm diameter 16 channel carbon fiber electrode arrays for single unit recordings. J Neural Eng. 2015;12(4)
6. Yang J, Martin DC. Microporous conducting polymers on neural microelectrode arrays: I Electrochemical deposition. Sensors and Actuators B: Chemical. 2004;101(1-2):133-142
Figures