2401
Comparison of inhomogeneous magnetization transfer imaging with myelin water imaging (MWI) and diffusion tensor imaging (DTI)1Radiology, University of Texas Southwestern Medical Center, Dallas, TX, United States, 2Radiology, Division of MR Research, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, United States, 3Philips Healthcare, Germany, 4Advanced Imaging Research Center, University of Texas Southwestern Medical Center, Dallas, TX, United States, 5Philips Healthcare, Gainesville, FL, United States
Synopsis
Inhomogeneous magnetization transfer (ihMT) imaging is an enhanced magnetization transfer technique, which has been shown to produce a higher white/gray matter contrast compared to conventional MT methods. This contrast is thought to be originating from dipolar order effects in myelinated tissues. In this study we compare ihMT with myelin water imaging and diffusion tensor imaging.
Introduction
Myelin imaging has been of great clinical interest because of its potential diagnostic value for various diseases including multiple sclerosis. Current efforts for myelin imaging include inhomogeneous magnetization transfer imaging (ihMT), which is recently introduced enhanced magnetization transfer imaging technique employing subtraction of dual off-resonance frequency saturation (alternating positive and negative) from a single one1,2,3,4. This technique has been shown to be more sensitive to myelinated tissue compared to the conventional magnetization transfer method and is suggested as a potential marker for myelin1. In this study, we investigate the sensitivity of ihMT to white matter microstructure and its specificity to myelin content by comparing ihMT with diffusion tensor imaging (DTI) and myelin water imaging (MWI) respectively.Methods
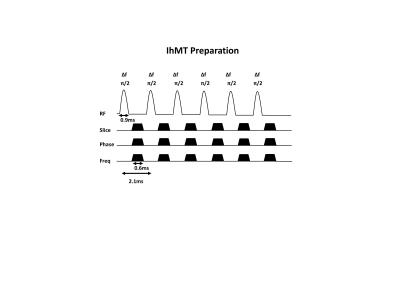
3 healthy volunteers (2 female, age: 33 ± 12 years) were scanned on a 3 Tesla Ingenia Philips MRI scanner (Philips Healthcare, Best, The Netherlands) equipped with a multi-transmit body coil and a 32-channel receive head-coil (Nova Medical Inc., Wilmington, MA, USA). A written informed consent was obtained from all volunteers and the study adhered to the local Institutional Review Board guidelines. The MRI scan protocol consisted of a 3D T1-weighted image acquistion (res=1x1x1mm3, TR/TE=8.1/3.7ms, FOV=224x224x160mm3, acquisition time=5min), DTI acquisition (res=1.75x1.75x2mm3, TR/TE= 4394/82ms, FOV=224x224x120mm3, one b=0 image and 32 diffusion-weighted images with a b-value of 1000 s/mm2, 2 averages, acquisition time=11min), MWI acquisition with a multi-echo 2D GRASE sequence (res=1x1x5mm3, 32 echoes, TR/TE/ΔTE=800/10/10ms, FOV=240x200x70mm3, flip angle=90°, acquisition time=9min), and a pulsed ihMT prepared 3D spoiled gradient echo (SPGR) acquisition (res=2.5x2.5x5mm3, TR/TE=70/1.6ms, FOV=220x220x120mm3, flip angle=10°, acquisition time=6 min 14 s, one reference image without saturation (M0) and 4 images with ihMT preparation with the following frequency offsets: +7 kHz (MT+), -7 kHz (MT-), alternating ±7 kHz (MT+-) and alternating -+7kHz (MT-+) respectively). The ihMT preparation was employed within each TR of the 3D SPGR and consisted of Hann-shaped RF pulses (6 pulses, pulse duration = 0.9 ms, flip angle = 90°, frequency offset (Δf) = ±7 kHz, RF phase cycling of 117°) followed by gaps and dephasing gradients as shown in Figure 1. This implementation insures long saturation times are achieved by the acquisition of the center of the k-space. The ihMT preparation length, TR and flip angles were optimized to achieve maximum ihMT effect.
Data post-processing: Fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD) and radial diffusivity (RD) maps were generated from motion corrected DTI image by the dtifit function in FMRIB's Diffusion Toolbox (FDT) of FMRIB Software Library (FSL). Myelin water fraction (MWF) was calculated on a voxel-wise basis for each subject by using a regularized non-negative least squares algorithm in a similar way to that described by Prasloski et al.5. The ihMT prepared images were co-registered to the reference image from the same subject and a custom FSL script was used compute ihMT ratio (ihMTR) per subject according to the following equation: ihMTR = (MT++ MT- -MT+- -MT-+)/M0. VBM8 software was used to calculate white matter (WM) and gray matter (GM) tissue probability maps based on T1-weighted images of each subject and to transform JHU white matters label atlas (ICBM-DTI-81) to T1-weighted image space for each subject. DTI, MWI and ihMT images were registered to T1-weighted image. The inverse transformation matrices were used to register WM and GM tissue probably maps and ICBM-DTI-81 to DTI, MWI and ihMT images. FA, MD, AD, RD and ihMTR maps were masked with WM and ICBM masks to calculate these values for various tracts.
Results and Discussion
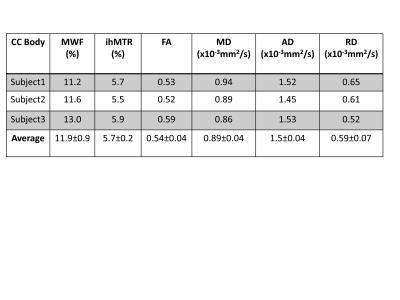
MWF, ihMTR and FA maps from a similar slice from the same subject is shown in Figure 2. The three methods use different underlying physics principles to create contrast: DTI is based on water diffusion, MWF on T2 variation while ihMT is based on the detection of the specific dipolar broadening profile. Our preliminary results indicate overall qualitative similarity between methods. Similar to MWF, ihMTR shows higher values in the cortiscospinal tract compared to the other white matter tracts. Figure 3 shows a preliminary comparison of the ihMTR with MWF and DTI metrics in the body of corpus callosum. All three methods display good consistency of measurements between subjects in this small preliminary sample. More subjects are being recruited to perform statistical correlations of ihMTR with MWF, FA, MD, RD and AD in various white matter tracts.Conclusions
This is the first study comparing ihMT with the other MRI methods, which are used to study white matter tissue microstructure. Our preliminary results suggest that ihMTR provides comparable contrast to MWF in the human brain white matter.Acknowledgements
No acknowledgement found.References
1. Varma, G., et al. Magnetization transfer from inhomogeneously broadened lines: A potential marker for myelin. Magn. Reson. Med. 2015; 73: 614–622.2. Girard, O. M., et al. Magnetization transfer from inhomogeneously broadened lines (ihMT): Experimental optimization of saturation parameters for human brain imaging at 1.5 Tesla. Magn. Reson. Med. 2015; 73: 2111–2121.3. Swanson S. D., et al. Molecular, dynamic, and structural origin of inhomogeneous magnetization transfer in lipid membranes. Magn. Reson. Med. 2016. DOI:10.1002/mrm.26210. 4. Varma, G. et al. Interpretation of magnetization transfer from inhomogeneously broadened lines (ihMT) in tissues as a dipolar order effect within motion restricted molecules. J. Magn. Reson. 2015; 260: 67–76. 5. Thomas Prasloski, Alexander Rauscher, Alex L. MacKay, Madeleine Hodgson, Irene M. Vavasour, Corree Laule, and Burkhard Mädler, 'Rapid Whole Cerebrum Myelin Water Imaging Using a 3d Grase Sequence', NeuroImage, 63 (2012), 533-39.Figures