2400
Neonatal MRI rotational motion correction using a wireless accelerometer (WiMoCo)1Academic Radiology, University of Sheffield, Sheffield, United Kingdom
Synopsis
A wireless accelerometer has been used to measure rotation angles on a dedicated neonatal MRI system. The measured angles have been used to help correct the k-space data to reduce ghosting artifacts. No interference between the accelerometer and the MR system was observed. Some limited improvement in ghosting was found but further work is required on the reconstruction algorithm. The device offers fast temporal resolution (10ms) and no sequence acquisition time overhead as it is a totally independent measurement system.
Introduction
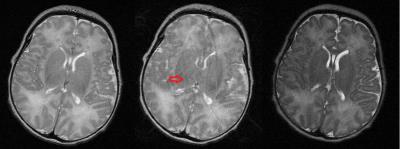
Neonatal MRI often suffers from extensive motion artifact, particularly when non-snapshot imaging sequences are required. A range of methods have been applied to correct neonatal motion including navigator pulses, optical tracking and auto-focus post-processing1,2. These can suffer from extended sequence time restricting minimum achievable repeat time, limited visual access and extended reconstruction times, respectively. This feasibility phantom study measured motion in real time using a wireless accelerometer to measure in-plane angular rotations which are common in neonatal imaging but difficult to correct with MR based navigator techniques. For example, images from the ’Firefly’ 3T dedicated neonatal MRI (Figure 1) installed on the NICU illustrate the problem. A Fast Spin Echo with a long acquisition time sometimes shows no motion artefact (Figure 2 Left) but often shows significant motion artifact (Figure 2 middle - arrow). A Single Shot Fast Spin Echo (SSFSE) (Figure 2 – right) shows no motion artefact but has lower SNR and resolutionMethods
A wireless accelerometer (Chronos-EZ430, TI) was used to measure rotation angles of a fruit phantom (orange) on dedicated 3T neonatal MRI system (Firefly, GE, Milwaukee, USA). The accelerometer was located beyond the magnet fringe field through a mechanical linkage and output was calibrated as a function of in-plane rotation angle about iso-centre3. Images were acquired with an axial SE (TR/TE= 300/15ms, SLT=3mm, In-plane=1mm, NEX=1) with and without manual rotation of the phantom through random angles (< +/-1 degrees). Raw data was acquired and signal for each phase step was assigned to new k-space locations to correct for the random rotations using Matlab. The wireless data was down-sampled and registered in time to match the separate k-space steps. The reconstruction matrix was extended to allow for rotations and image reconstruction was performed using a standard Fourier transform. No filtering was applied to the data set.Results
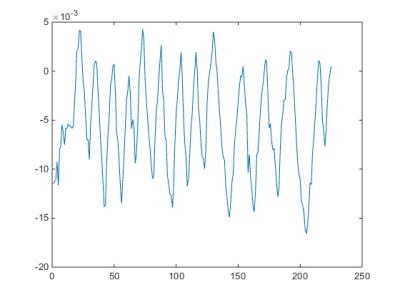
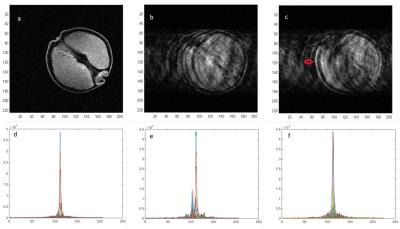
The wireless interface operated effectively during image acquisition. The device was not affected by the magnetic or radiofrequency fields applied during imaging (the device was kept outside the transmit coils) and did not produce any observable interference on the acquired images. This was to be expected given the widely different operating frequencies between the device (868 MHz) and the MR Larmor frequency used (128MHz). The USB interface receiving the wireless signal was extended partially into the RF screened room wave-guide to provide improved reception with the RF screened room door fully closed. Figure 3 shows measured in-plane rotation angle about iso-centre versus phase encoding step. Figure 4a shows the original image, Figure 4b the uncorrected image and Figure 4c the corrected image, yielding modest image quality improvement e.g. improvement in edge definition (red arrow) although significant ghosting is still observed. The k-space data sets, Figures 4d-f, underneath the corresponding images. Figure 4e shows the highly corrupted data set from the rotated phantom which is improved by the measured rotational correction as shown in Figure 4f, but not yet sufficiently to remove all the ghosting artifacts. Further work is in progress to improve the reconstruction algorithm.Discussion
The wireless accelerometer operated successfully in close proximity to the 3T neonatal MRI system. Rotation angles could be measured in real time and used retrospectively to help correct images for random in-plane rotations. The geometry and contrast of the randomly moving fruit was improved marginally with angular correction. Development of the reconstruction algorithm to further reduce ghosting is required including interpolation of the blank ‘pie-slice’ regions of k-space created by rotation. The difficulties of correcting a relatively simple planar rotation when the angles are known illustrates the complexity of neonatal MRI motion correction. The accelerometer provides full 3D motion information so could also be used to measure and additionally correct translational and through–plane motion. It also has great potential for use with prospective gating. The accelerometer in its current form has some low residual magnetism and so must be kept more than 100mm from the entry to the 3T magnet and linked to the object being imaged mechanically, which is not ideal.The battery is the most magnetic element of the accelerometer assembly and so in this study was located remotely through coaxial wiring. Non-magnetic batteries are relatively difficult to source and are expensive but are commercially available.The temporal resolution of the wireless accelerometer measurement is around 10ms so it has the potential to measure very rapid motion. In addition the motion measurement is completely independent from the image acquisition sequence so there is no additional acquisition sequence time burden. This study shows the overall feasibility of using a wireless accelerometer correction method for neonatal MRI motion artifacts.
Acknowledgements
We acknowledge the Wellcome Trust and GE Healthcare for providing the dedicated Neonatal MR system.References
1. Callaghan, M. F., Josephs, O., Herbst, M., Zaitsev, M., Todd, N., & Weiskopf, N. An evaluation of prospective motion correction (PMC) for high resolution quantitative MRI. Frontiers in Neuroscience, 2015, 9: 97.
2. Atkinson D, Hill D L G, Stoyle P N R, Summers P E and Keevil S F. "Automatic Correction of Motion Artefacts in Magnetic Resonance Images Using an Entropy Focus Criterion." IEEE Trans. Med. Imaging, 1997, 16(6): 903-910
3. Paley, M.N.; Ledger, A.; Leach, M.O.; Cummings, C.; Hughes, R.; Akgun, A. Wireless Accelerometer for MRI-Guided Interventional Procedures. Technologies 2013, 1: 44-53.
Figures