2399
Assessing Functional and Structural Connectivity in ex-Professional Athletes1School of Biomedical Engineering, McMaster University, Hamilton, ON, Canada, 2Department of Electrical and Computer Engineering, McMaster University, 3Department of Linguistics and Languages, McMaster University
Synopsis
Recently there has been considerable attention directed towards the increased risk for head injuries that athletes face while participating in high impact sports. Furthermore, there is also heightened interest in asymptomatic sub-concussive blows that possibly lead to long term neurological deficits. The goal of this study was to investigate retired professional athletes, who played at least 4 seasons of Canadian football, using functional connectivity mapping and DTI techniques. When compared to an age matched control population, differences were observed both in functional and structural connectivity, suggesting that even years after retiring the brain still exhibits signs of damage.
Introduction
Mild traumatic brain injury (mTBI), also known as concussion, affects upwards of 1.7 million people each year1. A concussive injury often comes with a host of post-concussive symptoms, ranging from fatigue, dizziness and headaches, to depression, irritability, deficits in memory and executive function2. In addition to these symptomatic concussive injuries, athletes participating in high impact contact sports (such as Canadian football and ice hockey) are prone to less symptomatic sub-concussive injuries, which may occur in large numbers throughout their career3. There is evidence which indicates that these repetitive sub-concussive blows place the athlete at higher risk for developing persistent post-concussive symptoms, structural alterations in the brain as well as neurodegenerative disease such as chronic traumatic encephalopathy (CTE)4,5. By applying advanced neuroimaging techniques on a population of retired professional athletes from the Canadian Football League (CFL) who had not been recently diagnosed for a mTBI, we sought to identify the presence of microstructural and functional alterations that may be the result of their high impact professional careers.Methods
Retired CFL players (n=10, mean age=56±6yrs) having played at least 4 seasons of professional football, and not having recently suffered a mTBI, were recruited for the study. Healthy subjects to serve as controls were sourced from online data repositories (Milwaukee, n=43, age=54±6yrs, ICBM, n=48, age=50±8yrs)6,7. A GE MR750 Discovery 3T MRI scanner and 32-channel RF receiver coil was used for scanning the retired athlete group. To assess functional connectivity, resting state functional BOLD data was acquired using an echo planar imaging (EPI) sequence (FOV=22cm, 64x64 matrix, flip angle=90o, TE/TR=35/2000ms, slice thickness=3mm and 175 temporal points). Axial diffusion tensor imaging (DTI) data was acquired using a dual echo EPI sequence (60 non-coplanar directions, TE/TR=87/8800ms, b=1000s/mm2, 122x122 matrix, 70 slices, 2mm thickness, FOV=244mm, ASSET=2, i.e. 2mm isotropic voxels). fMRI data was processed using the MELODIC toolbox within the FMRIB Software package8. Thirteen different activation networks were identified using probabilistic ICA. With the FMRIB Diffusion Toolbox (FDT) and Tract-Based Spatial Statistics (TBSS), diffusion tensors were reconstructed, a common registration target was created and each subjects aligned fractional anisotropy (FA) image was projected onto this target. Voxel wise statistics were performed, in addition to ROI analysis of 20 individual structures according to the JHU DTI-based white-matter atlas9. For fMRI and DTI data, group differences were probed through permutation testing methods (FSL randomise)10. This included the design of a simple general linear model, and application of Threshold-Free Cluster Enhancement (TFCE)11.Results
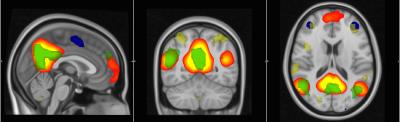
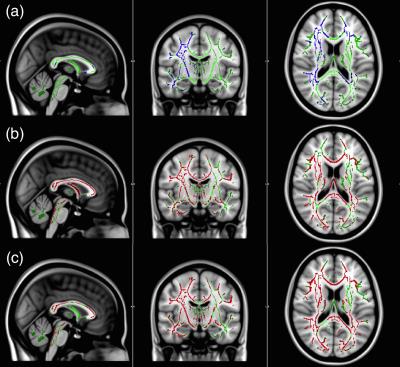
Interrupted functional connectivity within several different networks was observed. More specifically, decreases, relative to controls, were observed within the frontal lobe network, default mode network (DMN), cingulate network and executive control networks (p<0.05). Increased functional connectivity versus controls was also found in the DMN (p<0.05). DTI TBSS analysis identified deficits in FA in the corticospinal tracts, superior longitudinal fasciculi, thalamic nuclei, forceps minor and uncinate fasciculus (p<0.05) (Fig.1). ROI analysis of 22 white matter structures confirmed the visual results of TBSS, with significant decreases in FA (as well as increases in mean diffusivity, MD, and radial diffusivity, RD) observed in the anterior thalamic nuclei, corticospinal tracts, forceps minor, superior and inferior longitudinal fasciculus and uncinate fasciculus (p<0.05). Larger differences were observed in right brain white matter structures.Discussion
Our results suggest that even decades after retiring from participating in professional football, deficits in both structural and functional connectivity appear to remain within the brain. The anomalies we observed appear to have similar characteristics to those noted weeks to months after a mTBI12. Decreased FA was observed through TBSS across several white matter structures, whereas general ROI analysis provided similar results (also increased MD and RD), and identified increased deficits on the right side of the brain. Hyper-connectivity of the DMN pathway and recruitment of surrounding structures could be interpreted as compensation for the loss of core white matter microstructural integrity.Conclusion
There are significant deficits in both resting state functional network connectivity and core microstructural integrity present in retired professional football athletes, even decades after professional play.Acknowledgements
The authors would like to thank Steve Buist and Drew Edwards from the Hamilton Spectator for support in this project.References
1. Faul M, Xu L, Wald MM, Coronado VG. (2010). Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. http://doi.org/10.1016/B978-0-444-52910-7.00011-8
2. Stern, R. A., Riley, D. O., Daneshvar, D. H., Nowinski, C. J., Cantu, R. C., & Mckee, A. C. (2011). Long-term Consequences of Repetitive Brain Trauma: Chronic Traumatic Encephalopathy, 3(October), 460–467. http://doi.org/10.1016/j.pmrj.2011.08.008
3. Talavage, T. M., Nauman, E. A., Breedlove, E. L., Yoruk, U., Dye, A. E., Morigaki, K. E., … Leverenz, L. J. (2014). 2 3 5 6, 338, 327–338. http://doi.org/10.1089/neu.2010.1512
4. McKee, A. C., Cantu, R. C., Nowinski, C. J., Hedley-Whyte, E. T., Gavett, B. E., Budson, A. E., … Stern, R. a. (2009). Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. Journal of Neuropathology and Experimental Neurology, 68(7), 709–35. http://doi.org/10.1097/NEN.0b013e3181a9d503
5. Tartaglia, M. C., Hazrati, L. N., Davis, K. D., Green, R. E., Wennberg, R., Mikulis, D., ... & Tator, C. (2014). Chronic traumatic encephalopathy and other neurodegenerative proteinopathies. Frontiers in human neuroscience,8, 30.
6. International Neuroimaging Data-Sharing Initiative. (2009). 1000 Functional Connectomes Project. Retrieved from http://fcon_1000.projects.nitrc.org/
7. Laboratory of Neuroimaging. (2011). International Consortium for Brain Mapping. Retrieved from https://ida.loni.usc.edu/
8. Jenkinson, M., Beckmann, C. F., Behrens, T. E., Woolrich, M. W., & Smith, S. M. (2012). Fsl. Neuroimage, 62(2), 782-790.
9. Mori et al., MRI Atlas of Human White Matter. Elsevier, Amsterdam, The Netherlands (2005).
10. Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. NeuroImage, 2014;92:381-397.
11. Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TEJ. (2006). Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 31(4):1487-1505.
12. Sharp, D. J., Beckmann, C. F., Greenwood, R., Kinnunen, K. M., Bonnelle, V., Boissezon, X. De, … Leech, R. (2011). connectivity after traumatic brain injury. http://doi.org/10.1093/brain/awr175
Figures