2398
Traumatic Spinal Cord Injury Induces Cortical Diffusion MRI Changes1Radiology, Washington University in Saint Louis, Saint Louis, MO, United States, 2Neurological Surgery, Washington University in Saint Louis, Saint Louis, MO, United States, 3Washington University in Saint Louis, Saint Louis, MO, United States
Synopsis
Spinal cord injury (SCI) is a significant public health problem. A major shortcoming limiting efforts to improve the treatment of SCI is the lack of quantifiable metrics on which to base clinical decisions. In current study, we have utilized diffusion basis spectrum imaging (DBSI) to more accurately differentiate and quantify axonal injury, demyelination, inflammation and edema/tissue loss. DBSI results suggest chronic SCI does result in axonal injury and edema/tissue loss at the level of the cerebral peduncle. These results demonstrated axons may be preserved rostral to the site of injury, and may provide some insight as to why some patients respond to more recent epidural stimulation even years out from injury.
Introduction
Promising treatments are being developed to promote functional recovery after SCI. Methods to quantify Wallerian degeneration of damaged axons and demyelination of intact axons following SCI are essential to guide regenerative therapies. A novel data-driven model-selection algorithm known as Diffusion Basis Spectrum Imaging (DBSI) has been proposed to more accurately delineate white matter injury.1,2 The objective of this study was to investigate whether DTI/DBSI changes that extend to level of the cerebral peduncle (CP) and internal capsule (IC) following a SCI could be correlated with clinical function.Methods
Human Subjects
Procedures involving human subjects were approved by the Institutional Review Board of Washington University. Every subject provided informed consent before their participation in the study. A prospective non-randomized cohort of twenty-three patients with chronic spinal cord injuries (Five cervical ASIA A patients, one cervical ASIA B patient, nine cervical ASIA D patients, four thoracic ASIA A patients, one thoracic ASIA B, one thoracic ASIA C, and two thoracic AISA D) and seventeen control subjects underwent cranial diffusion weighted imaging, followed by whole brain DTI and DBSI computations. Clinical grading of patient functional status was made with International standards for neurological classification of spinal cord injury (ISNCSCI) as modified by the American Spinal Injury Association (ASIA) Scale.3
DBSI
Magnetic resonance images were acquired using a 3 T scanner (Trio, Siemens, Erlangen, Germany) using a single-shot diffusion weighted SE-EPI sequence with the following parameters: TR = 10000 ms; TE = 120 ms; FOV = 256×256 mm2; acquisition matrix = 128 × 128; slice thickness = 2 mm; in-plane resolution = 2 × 2 × 2 mm3; acquisition time = 15 minutes. Images from all subjects were registered to standard JHU-DTI-MNI single-subject atlas (also known as the “Eve Atlas”). DTI and DBSI post-processing were conducted using software developed by Matlab.
TBSS
The whole brain voxel-wise analysis of DTI and DBSI were carried out using Tract Based Spatial Statistics (TBSS)4, part of FSL5. DTI and DBSI indices were projected onto this skeleton for statistical analyses. Nonparametric permutation tests were used for voxel-wise statistical analysis of the skeletons between health controls and SCI patients. The significance threshold for group differences was set at P < 0.05.
Data analysis
Standard Regions of Interest (ROIs) of CP and IC for each participant were selected based on the Johns Hopkins University (JHU) MNI template White Matter Parcellation Map. Three subgroups of patients (Cervical A/B, Cervical D and Thoracic A/B/C/D) were included in the region-based analysis. Tract-Based Spatial Statistics (TBSS) was also applied to allow whole-brain white matter analysis between controls and all patients. Student’s t-test was used for statistical analysis of group difference.
Results
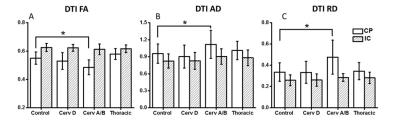
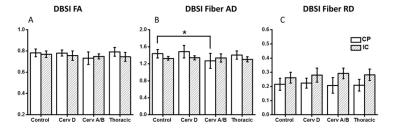
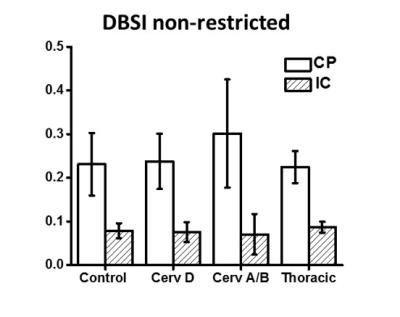
Tract-based spatial statistics comparison of all SCI cohorts with healthy controls demonstrated no significant differences. DTI indices (FA, AD and RD) from ASIA-A/B patients are different from controls only in CP region (Fig. 1). FA values were significantly lower at the level of CP. In contrast, RD and AD values were both elevated at the level of the CP as compared to normal controls. As demonstrated in Fig. 2, DBSI showed decreased FA values in the CP from cervical ASIA D to ASIA A/B, but did not reach significance as with DTI. AD values of ASIA A/B patients were significantly lower in CP, while RD values not different, compared to controls. In contrast to DTI, edema/tissue loss can be accounted for with DBSI measures. As depicted in Figure 3, increased non-restricted fraction in the CP contributed to the elevations of both DTI AD and RD, which also explains the over-estimated DTI FA drop in CP.Conclusions
Cervical ASIA A/B SCI patients had higher levels of axonal injury and edema/tissue loss as measured by DBSI at the level of the CP. DTI was able to detect differences in cervical ASIA A/B patients (FA, AD and RD), but were non-specific to pathologies. Increased water fraction indicated by DBSI non-restricted isotropic diffusion fraction in the CP, explains the simultaneously increased DTI AD and DTI RD values. Our results further demonstrate the utility of DTI to detect disruption in axonal integrity in white matter, yet incapable of differentiating true axonal injury from inflammation/tissue loss. Our results suggest a preservation of axonal integrity at the cortical level and has implications for future regenerative clinical trials.Acknowledgements
Supported by the NIH R01-NS047592, P01-NS059560, K23-NS084932 and The Missouri Spinal Cord Injuries Research Program.References
[1] Wang, Y., et al., Differentiation and quantification of inflammation, demyelination and axon injury or loss in multiple sclerosis. Brain, 2015. 138(Pt 5): p. 1223-38.
[2] Wang, Y., et al., Quantification of increased cellularity during inflammatory demyelination. Brain, 2011. 134(Pt 12): p. 3590-601.
[3] Kirshblum S, Waring W, 3rd: Updates for the International Standards for Neurological Classification of Spinal Cord Injury. Phys Med Rehabil Clin N Am 25:505-517, vii, 2014
[4] Smith, S.M., et al., Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31.4 (2006): 1487-1505.
[5] Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al: Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23 Suppl 1:S208-219, 2004
Figures