2396
Imaging of thalamic calcium deposits due to sports-related concussion using Quantitative Susceptibility Mapping (QSM)1Buffalo Neuroimaging Analysis Center, Department of Neurology, Jacobs School of Medicine and Biomedical Sciences, University at Buffalo, The State University of New York, Buffalo, NY, United States, 2MRI Clinical and Translational Research Center, Jacobs School of Medicine and Biomedical Sciences, University at Buffalo, The State University of New York, Buffalo, NY, United States, 3Department of Psychiatry, Jacobs School of Medicine and Biomedical Sciences, University at Buffalo, The State University of New York, Buffalo, NY, United States, 4Department of Neurosurgery, Jacobs School of Medicine and Biomedical Sciences, University at Buffalo, The State University of New York, Buffalo, NY, United States, 5Department of Orthopaedics, Jacobs School of Medicine and Biomedical Sciences, University at Buffalo, The State University of New York, Buffalo, NY, United States
Synopsis
This work explored the hypothesis that the activation of N-methyl-D-aspartate (NMDA)-receptors in the thalamus results in persistent calcium deposits after a sports-related concussion that can be visualized clinically with Quantitative Susceptibility Mapping at 3 Tesla. The study involved 22 retired professional contact-sports athletes and 45 controls. We found a significantly higher incidence of thalamic micro-calcifications in contact-sports athletes compared to controls, in particular in ice hockey players.
Introduction
Intracellular calcium influx is a key event in the pathophysiology of concussion1. Animal experiments have demonstrated a continuing, delayed influx of Ca2+ into the thalamus for 48 hours and longer after the injury2. Preliminary data using the fluid percussion injury model of traumatic brain injury [submitted to this conference] suggest that the strong diamagnetism of calcium phosphates3-5 allows in vivo visualizing elevated thalamic calcium concentrations with the novel phase-based technique Quantitative Susceptibility Mapping (QSM)6-9.
In the present work, we explored the hypothesis that sports-related concussion results in persistent thalamic calcium deposits that can be visualized clinically with QSM at 3 Tesla.
Methods
Subjects: This IRB-approved study included 68 subjects (no females); 22 retired professional contact-sports athletes (15 ice hockey and 7 American football players; 55.5±11.2 years), 20 professional athlete controls (retired non-contact sports athletes: cycling, running, skiing, swimming, triathlete; 57.2±9.8 years), and 25 normal controls (non-professional, never played contact sports, self-reported; 55.1±9.9 years). All three groups were age- and sex-matched.
Data acquisition and image reconstruction: Participants were imaged at 3T (GE Signa Excite HD 12.0) with an eight-channel head-and-neck coil using a 3D gradient-echo (GRE) sequence (matrix 512x192x64, 256x192x128mm3, TE/TR=22ms/40ms, BW=13.9kHz, tip=12°). We reconstructed magnetic susceptibility maps from k-space using scalar-phase-matching10,11, gradient unwarping12, best-path unwrapping13, V-SHARP14-16, and HEIDI17. Also, we reconstructed Susceptibility Weighted Images (SWI) and filtered phase images18,19.
Analysis: A trained image analyst outlined thalamic calcifications based on the following definition: Focal, hyperintense region in the thalamus on susceptibility maps, associated with a co-localized intensity variation on phase images (to avoid over-interpretation of QSM-related artifacts). For every identified lesion we determined the lesion’s volume and average magnetic susceptibility. If a calcification was identified, the analyst inspected both GRE magnitude and SWI for intensity variations at the location of the lesion. Differences in the incidence of thalamic calcifications between groups were tested using Pearson Chi-Square test. A p-value below 0.05 was considered as statistically significant.
Results
We found thalamic micro-calcifications in 23% (N=5; -48.5±23.5ppb; 22.5±10.6mm3) of contact-sports athletes, 10% of athlete controls (N=2; -50.6±6.9ppb; 18.5±7.0mm3), and none in the normal controls. The incidence of micro-calcification was significantly different between groups (p=0.039). All contact-sports athletes with calcifications were ice hockey players (100%); none of the football players showed calcifications. Athlete controls with lesions were runners/triathletes.
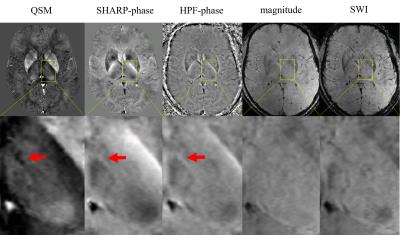
All lesions were found in the medial nuclear group of the thalamus; one athlete control had lesions in the anteroventral and reticular nuclei. All lesions appeared hypointense on both high-pass filtered and SHARP phase images but were substantially better defined on QSM. None of the lesions could be identified on either magnitude images or SWI. Lesions in contact-sports athletes were found bilaterally (2) or only in the left (2) and right (1) thalami, respectively. Lesions in athlete controls were found bilaterally. Figure 1 shows images of an exemplary subject with thalamic calcification.
Discussion
QSM revealed pathology that was not discernible on conventional magnitude-based images, despite the use of a clinical pulse sequence that had not been optimized for QSM. The lack of magnitude lesion contrast indicates a diffuse microscopic distribution of the calcium (potentially intracellular), compared to agglomeration in a solid calcium core. The presence of thalamic calcification years after acute concussions indicates that calcium deposits are persistent over time.
Calcium deposition may be related to the activation of N-methyl-D-aspartate (NMDA)-receptors in the thalamus following concussion1, which results in abnormally high levels of intracellular Ca2+.20 Mitochondria buffer large amounts of calcium and may undergo a Ca2+-induced permeability transition. The free calcium is sequestered in CaHPO4 and practically insoluble Ca3(PO4)2,21 which may be persistent. Ice hockey players may be more affected by concussion-related pathology than football players because collision speeds are higher in hockey and they commonly play more games per season with longer average activity times per game.
Conclusion
QSM detects concussion-related thalamic pathology not visible with conventional, magnitude-based MRI. Future research will investigate the potential of visualizing calcium phosphates in vivo as a biomarker for studying concussion-related pathophysiology and clinical assessment of brain injury.Acknowledgements
Research reported in this publication was funded by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR001412. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.References
[1] M. C. Choe, “The Pathophysiology of Concussion,” Curr Pain Headache Rep, vol. 20, no. 6, p. 42, 2016.
[2] C. L. Osteen, A. H. Moore, M. L. Prins, and D. A. Hovda, “Age-dependency of 45calcium accumulation following lateral fluid percussion: acute and delayed patterns.” J Neurotrauma, vol. 18, no. 2, pp. 141–62, 2001.
[3] S. Straub, F. B. Laun, J. Emmerich, B. Jobke, H. Hauswald, S. Katayama, K. Herfarth, H.-P. Schlemmer, M. E. Ladd, C. H. Ziener, D. Bonekamp, and M. C. Röthke, “Potential of quantitative susceptibility mapping for detection of prostatic calcifications,” J Magn Reson Imaging, 2016.
[4] W. Chen, W. Zhu, I. Kovanlikaya, A. Kovanlikaya, T. Liu, S. Wang, C. Salustri, and Y. Wang, “Intracranial calcifications and hemorrhages: characterization with quantitative susceptibility mapping.” Radiology, vol. 270, no. 2, pp. 496–505, 2014.
[5] F. Schweser, A. Deistung, B. W. Lehr, and J. R. Reichenbach, “Differentiation Between Diamagnetic and Paramagnetic Cerebral Lesions Based on Magnetic Susceptibility Mapping,” Med Phys, vol. 37, no. 10, pp. 5165–5178, 2010.
[6] C. Liu, W. Li, K. A. Tong, K. W. Yeom, and S. Kuzminski, “Susceptibility-weighted imaging and quantitative susceptibility mapping in the brain,” J Magn Reson Imaging, vol. 42, no. 1, pp. 23–41, 2015.
[7] E. M. Haacke, S. Liu, S. Buch, W. Zheng, D. Wu, and Y. Ye, “Quantitative susceptibility mapping: current status and future directions,” Magn Reson Imaging, vol. 33, no. 1, pp. 1–25, 2015.
[8] J. R. Reichenbach, F. Schweser, B. Serres, and A. Deistung, “Quantitative Susceptibility Mapping: Concepts and Applications,” Clin Neuroradiol, vol. 25, no. S2, pp. 225–230, 2015.
[9] Y. Wang and T. Liu, “Quantitative susceptibility mapping (QSM): Decoding MRI data for a tissue magnetic biomarker,” Magn Reson Med, vol. 73, no. 1, pp. 82–101, 2015.
[10] J. A. de Zwart, P. J. Ledden, P. Kellman, P. van Gelderen, and J. H. Duyn, “Design of a SENSE-optimized high-sensitivity MRI receive coil for brain imaging.” Magn Reson Med, vol. 47, no. 6, pp. 1218–27, 2002.
[11] K. E. Hammond, J. M. Lupo, D. Xu, M. Metcalf, D. A. C. Kelley, D. Pelletier, S. M. Chang, P. Mukherjee, D. B. Vigneron, and S. J. Nelson, “Development of a robust method for generating 7.0 T multichannel phase images of the brain with application to normal volunteers and patients with neurological diseases.” NeuroImage, vol. 39, no. 4, pp. 1682–1692, 2008.
[12] P. Polak, R. Zivadinov, and F. Schweser, “Gradient Unwarping for Phase Imaging Reconstruction,” ISMRM 2015, p1279.
[13] H. S. Abdul-Rahman, M. A. Gdeisat, D. R. Burton, M. J. Lalor, F. Lilley, and C. J. Moore, “Fast and robust three-dimensional best path phase unwrapping algorithm.” Appl Opt, vol. 46, no. 26, pp. 6623–35, 2007.
[14] F. Schweser, A. Deistung, B. W. Lehr, and J. R. Reichenbach, “Quantitative imaging of intrinsic magnetic tissue properties using MRI signal phase: An approach to in vivo brain iron metabolism?” NeuroImage, vol. 54, no. 4, pp. 2789–2807, 2011.
[15] P. S. Özbay, A. Deistung, X. Feng, D. Nanz, J. R. Reichenbach, and F. Schweser, “A comprehensive numerical analysis of background phase correction with V-SHARP,” NMR Biomed (epub).
[16] B. Wu, W. Li, A. Guidon, and C. Liu, “Whole brain susceptibility mapping using compressed sensing.” Magn Reson Med, vol. 24, pp. 1129–36, 2011.
[17] F. Schweser, K. Sommer, A. Deistung, and J. R. Reichenbach, “Quantitative susceptibility mapping for investigating subtle susceptibility variations in the human brain.” NeuroImage, vol. 62, no. 3, pp. 2083–2100, 2012.
[18] J. R. Reichenbach and E. M. Haacke, “High resolution BOLD venographic imaging: a window into brain function,” NMR Biomed, vol. 14, no. 7-8, pp. 453–467, 2001.
[19] E. M. Haacke, Y. Xu, Y.-C. N. Cheng, and J. R. Reichenbach, “Susceptibility weighted imaging (SWI).” Magn Reson Med, vol. 52, no. 3, pp. 612–8, 2004.
[20] C. L. Osteen, C. C. Giza, and D. A. Hovda, “Injury-induced alterations in N-methyl-D-aspartate receptor subunit composition contribute to prolonged 45calcium accumulation following lateral fluid percussion,” Neuroscience, vol. 128, no. 2, pp. 305–322, 2004.
[21] A. V. Panov, L. Andreeva, and J. T. Greenamyre, “Quantitative evaluation of the effects of mitochondrial permeability transition pore modifiers on accumulation of calcium phosphate: Comparison of rat liver and brain mitochondria,” Archives of Biochemistry and Biophysics, vol. 424, no. 1, pp. 44–52, 2004.
Figures