2393
A Repetitive Traumatic Brain Injury Model Characterized with Diffusion Tensor and Diffusion Kurtosis Imaging with Neuropathological Correlation1Center for Neuroscience and Regenerative Medicine, Uniformed Services University of the Health Sciences, Bethesda, MD, United States, 2Anatomy, Physiology and Genetics, Uniformed Services University of the Health Sciences, Bethesda, MD, United States, 3Department of Psychiatry, University of Maryland School of Medicine at Baltimore, Baltimore, MD, 4Radiology, University of New Mexico, Albuquerque, NM, United States, 5Radiology and Radiological Sciences, Uniformed Services University of the Health Sciences, Bethesda, MD
Synopsis
Mild traumatic brain injury (mTBI) often involves single (s-mTBI) or repetitive (r-mTBI) head injury, which may differ in the potential for long term symptoms and chronic neurodegeneration. Non-invasive approaches that can detect damage and predict outcome are a high priority for clinical care of mTBI patients. Magnetic resonance (MR) diffusion tensor imaging (DTI) and diffusion kurtosis imaging (DKI) techniques can identify microstructural changes associated with Gaussian (DTI) and non-Gaussian (DKI) water diffusion properties. Mouse models of s-mTBI and r-mTBI targeting anterior brain regions (impact site at bregma) were developed for longitudinal MRI studies with corresponding neuropathology.
Purpose
To utilize DTI and DKI for improved
characterization of murine models of single or repetitive traumatic brain
injury, and to correlate DTI and DKI data with neuropathological changes in mice.
Materials and Methods
TBI Induction: For single mild TBI (s-mTBI), 8-10 week old male C57BL/6 mice had a scalp incision to expose the skull and received a stereotaxically controlled impact (3 mm tip) at bregma (1.5 mm depth; 4.0 m/sec; 100 msec dwell time). For repetitive mild TBI (r-mTBI), mice received a milder impact (1.0 mm depth; 4.0 m/sec; 200 msec dwell time) onto the scalp over bregma each day for 5 days. The r-mTBI times are after last impact.
MRI protocol: In vivo MR imaging was performed on a 7T small animal Bruker BioSpec (Bruker Biospin , Billerica, MA) scanner equipped with 650 mT/m gradient coils to evaluate longitudinal changes in both the corpus callosum and the overlying cortical gray matter after s-mTBI (n = 6) and r-mTBI (n = 12). DTI scans were performed at baseline and at 3, 6, and 42 days post-TBI/sham. Animals were anaesthetized with 1% isofluorane and placed in a magnetic resonance-compatible head holder. 2D single-shot echo planar imaging (EPI) (TR/TE= 4500/30 msec; 2 repetitions) was used to acquire 4-b = 0 s/mm2 images and 7 diffusion weighted images (b = 400, 800,…., 2800 s/mm2) using a Stejskal-Tanner [1] diffusion preparation with Δ=12 msec and δ=5 msec; field-of-view=14×14 mm2, matrix=80×80, and slice thickness=0.75 mm. Regions-of-interest (ROIs) in the corpus callosum (CC) and cerebral cortex were manually drawn on 5 consecutive slices (one at the bregma level slice, one rostral and three caudal to bregma level). ROIs for the medial cortex were drawn from the midline to above the cingulum laterally. DTI data were analyzed using TORTOISE[2] and FSL[3] software. Fractional anisotropy (FA), mean, axial, and radial diffusivity (MD, AD, and RD) maps were calculated. The corpus callosum and medial cortex ROIs were overlaid onto DTI maps for further quantitative analysis. DKI data were analyzed using adapted Matlab software to calculate mean kurtosis (MK), axial kurtosis (Ka) and radial kurtosis (Kr) maps.
Histological analysis: Fluorescence immunohistochemistry for b-APP, CD11b, GFAP and MOG was used to detect damaged axons, microglia activation, astrogliosis and demyelination, respectively. Prussian blue histological stain was used to detect hemosiderin as evidence of prior bleeds.
Results and Discussion
T2-weighted images at the impact site did not show signs of edema or overt structural damage in either the corpus callosum or cortex. Consistent with this finding, there was no positive Prussian blue staining in either the corpus callosum or cortex in sham or mTBI mice at any time point examined.
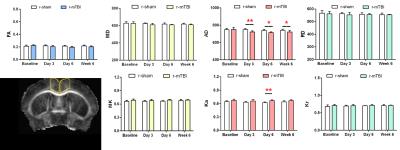
In the corpus callosum, after s-mTBI, FA decreased at 3, 6 and 42 days at the impact site after injury. No significant changes were noticed in MD or AD, while RD increased at day 6 after injury (Figure 1). After r-mTBI, no significant changes were seen in any DTI or DKI parameters in the corpus callosum at the impact site. This is also reflected by the small but significant decrease in myelination in the corpus callosum as illustrated with myelin-olgodendrocyte glycoprotein immunohistochemistry.
In the medial cerebral cortex, after s-mTBI, no significant changes were seen in any DTI or DKI parameters at the site of impact. However, after r-mTBI, DTI showed decreased AD in the medial cortex site at 3, 6, and 42 days after last impact. Ka increased at 6 days after r-mTBI (Figure 2). Both gaussian (AD) and non-gaussian (Ka) axial components indicate abnormalities in the cortex. Reflecting these MRI measures, in the cortex of the r-mTBI mice there was a significant increase in the area of CD11b immunolabeling and in the percentage of activated microglia, which was not found in s-mTBI or s-sham mice.
Astrogliosis was not significantly different in the cortex for either model. Axon damage and neuroinflammation in the corpus callosum is milder in the r-mTBI than in s-mTBI, which was previously characterized [4, 5].
Therefore, DTI indicates milder injury in the corpus callosum in the repetitive injury model, as compared to the single impact TBI while DTI/DKI reveals cortical changes in only the r-mTBI model.
Conclusion
Repetitive mild TBI produces
cortical abnormalities detectable by DTI. This finding is supported by tissue
analysis showing a significant increase of microglial activation in the cortex
after repetitive mild TBI, along with axon damage, and behavioral deficits
associated with the corresponding cortex. DTI was more sensitive than DKI for
both white matter and cortical abnormalities in these mild forms of TBI. Investigation of grey as well as the
typically studied white matter is warranted for imaging of traumatic brain
injury.
Acknowledgements
Supported by the U.S. Department of Defense in the Center for Neuroscience and Regenerative Medicine (CNRM), with technical support from the CNRM Translational Imaging Facility and Pre-clinical Core.We thank Drs. R.P. Gullapalli and J. Zhuo (University of Maryland, Baltimore, MD, USA) for providing the MATLAB code for DKI processing.References
1. Stejskal, E.O. and J.E. Tanner, Spin diffusion measurements: Spin echoes in the presence of a time dependent field gradient. J. Chem Phys, 1965. 42: p. 288-292.
2. Pierpaoli, C., L. Walker, M.O. Irfanoglu, et al. Tortoise: an integrated software package for processing of diffusion MRI data. in International Society of Magnetic Resonance in Medicine. 2010. Stockholm, Sweden.
3. Smith, S.M., M. Jenkinson, M.W. Woolrich, et al., Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage, 2004. 23 Suppl 1: p. S208-19.
4. Sullivan, G.M., A.J. Mierzwa, N. Kijpaisalratana, et al., Oligodendrocyte lineage and subventricular zone response to traumatic axonal injury in the corpus callosum. J Neuropathol Exp Neurol, 2013. 72(12): p. 1106-25.
5. Mierzwa, A.J., C.M. Marion, G.M. Sullivan, et al., Components of myelin damage and repair in the progression of white matter pathology after mild traumatic brain injury. J Neuropathol Exp Neurol, 2015. 74(3): p. 218-32.
Figures