2392
Longitudinal changes of neurovascular responses to breathhold challenge in patients with moderate traumatic brain injury1Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Charlestown, MA, United States, 2Department of Dermatology, Massachusetts General Hospital, Boston, MA, United States, 3Division of Neuroradiology, Department of Radiology, Massachusetts General Hospital, Boston, MA, United States, 4Department of Emergency Medicine, Massachusetts General Hospital, Boston, MA, United States, 5Department of Cognitive, Linguistic and Psychological Sciences, Brown University, Providence, RI, United States
Synopsis
There is an increasing evidence that neurovascular dysregulation contributes to the persistent symptoms in patients with traumatic brain injury (TBI). Damaged microvasculature may disrupt the neurovascular coupling, especially under physiological stress, where the local cerebral blood flow (CBF) no longer matches the metabolic requirements of the tissue. Our findings of negative or abnormally delayed blood oxygenation level dependent (BOLD) signal changes in response to breathhold challenge can potentially be used as an imaging marker to localize subtle abnormal vascular function in individual patients with moderate TBI. The restoration of abnormally delayed BOLD responses at chronic stage suggest that such an imaging marker may be used to follow up the patients.
Introduction
There is increasing evidence that neurovascular dysregulation
contributes to the persistent symptoms in patients with traumatic brain injury
(TBI). In a recent animal study, the cerebral vasculature was reported to
be more sensitive to blast-induced TBI than other elements in the brain 1. Damaged
microvasculature may disrupt neurovascular coupling, especially under
physiological stress, where the local cerebral blood flow no longer matches the
metabolic requirements of the tissue. The negative or abnormally delayed blood
oxygenation level dependent (BOLD) signal changes under hypercapnic challenge,
recorded in our published 2 and recent
preliminary findings in both patients 3 and animals 4 with mTBI,
suggest a potentially novel imaging marker with a sensitivity to localize
subtle abnormal vascular function in individual TBI patients. Such an imaging
marker may be used to characterize the persistent post-traumatic symptoms that
persist for a long time after TBI. In the present study, instead of
administering low-dose exogenous carbon dioxide, we applied breathhold challenge
for the hypercapnic fMRI on patients with moderate TBI within 48 hours, 2 weeks
and 3 months after injury.Subjects and Methods
Participants: Eleven patients
with moderate TBI (5 males, 6 females, aged from 21 to 79 years) were
included. All the patients required hospital admission because of the
head injury. The Glasgow Coma Scale scored above 9 at admission and
the patients also had abnormal imaging findings. Methods: MRI scanning
was performed on a 3-Tesla scanner (Siemens Medical Germany) in the Athinoula
A. Martinos Center for Biomedical Imaging at the Massachusetts General
Hospital. Each patient was scanned within 48 hours (Scan1), 2 weeks
(Scan2) and 3 months (Scan3) after the injury All the experimental procedures
were explained to the subjects, and signed informed consent was obtained prior
to participation in the study. Whole brain MRI datasets were acquired for
each subject: 1) standard high-resolution sagittal images acquired with
volumetric T1-weighted 3D-MEMPRAGE (TR=2530ms, TE=1.69ms/3.55ms/5.41ms/7.27ms,
flip angle=7º, FOV=256mm, matrix=256´256, slice thickness=1mm); 2) SMS
BOLD-fMRI images acquired with gradient-echo echo planar imaging (EPI) sequence
(TR=1250ms, TE=30ms, flip angle=90º, FOV=256mm, matrix=108´108,
thickness=2.4mm) while the subject had breathhold challenge. The paradigm
consisted of 2 consecutive phases (resting and breathhold) repeated 5 times.
The resting phase lasted no less than 60 seconds, while the breathold phase
lasted 30 seconds. The challenge lasted 10 minutes. Data analysis: All the BOLD-fMRI
data were imported into the software Analysis of Functional NeuroImage (AFNI) 5 (National
Institute of Mental Health, http://afni.nimh.nih.gov) for time-shift
correction, motion correction, normalization and detrending. Individual maps of
neurovascular response with percent BOLD signal change were derived.
Breathhold index (BHI) was derived using multiple regression with a regressor
of breath duration. BHI was defined by the maps of total percent BOLD
signal changes over per unit time of breath. Analysis of statistical parametric
maps were corrected at the overall threshold of p<0.05. The amplitude
of positive BHI change in each brain region was derived based on the
parcellation using Freesurfer 6
(http://surfer.nmr.mgh.harvard.edu). To increase the sensitivity of detecting
negative or abnormally delayed BOLD signal changes, Hilbert transform analysis 7
was applied to derive individual maps with BOLD response delay relative to the
changes of breath duration. Results
Reduced amplitude of BHI was observed in all brain regions in 2-week
follow-up (Scan2) relative to the 48-hour initial (Scan1) and 3-month follow-up
scan (Scan3) (Figure 1), which is consistent with the reduced cerebral
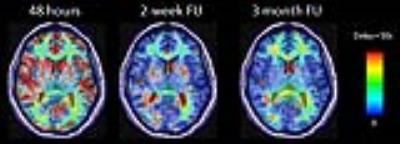
perfusion that we reported in the same group of patients. Figure 2 shows
abnormally delayed BOLD signal changes to the breathhold challenge in multiple
brain regions of a representative patient in the 48-hour scan. In the
2-week and 3-month follow-ups, the BOLD responses to the breathhold challenge
in some of these brain regions restored to normal.Discussion
The abnormally delayed BOLD responses in prior hypercapnic studies have
been reported in patients with steno-occulsive disease 7 as well as
in patients with mild traumatic brain injury 3; in those
individuals, it was suggested that the delayed response was due to a relative
“steal phenomenon” of compromised tissue away from intact areas. The
parallel reduction of cerebral perfusion and BHI suggest the potential
disruption of neurovascular coupling due to reduced cerebral blood flow.
Our findings suggest that the neurovascular response to the hypercapnic
breathhold challenge may be used as an imaging marker to localize and follow
subtle abnormal vascular function.Acknowledgements
This research was supported by Congressionally Directed Medical Research Program W81XWH-13-2-0067.References
1. Sosa MA, De Gasperi R, Paulino AJ, et al. Blast overpressure induces shear-related injuries in the brain of rats exposed to a mild traumatic brain injury. Acta Neuropathol Commun 2013;1:51.
2. Chan ST, Evans KC, Rosen BR, Song TY, Kwong KK. A case study of magnetic resonance imaging of cerebrovascular reactivity: A powerful imaging marker for mild traumatic brain injury. Brain injury 2014:1-5.
3. Mutch WA, Ellis MJ, Ryner LN, et al. Brain magnetic resonance imaging CO2 stress testing in adolescent postconcussion syndrome. J Neurosurg 2016;125:648-660.
4. Long JA, Watts LT, Li W, et al. The effects of perturbed cerebral blood flow and cerebrovascular reactivity on structural MRI and behavioral readouts in mild traumatic brain injury. J Cereb Blood Flow Metab 2015;35:1852-1861.
5. Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research 1996;29:162-173.
6. Destrieux C, Fischl B, Dale A, Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage 2010;53:1-15.
7. Poublanc J, Han JS, Mandell DM, et al. Vascular steal explains early paradoxical blood oxygen level-dependent cerebrovascular response in brain regions with delayed arterial transit times. Cerebrovasc Dis Extra 2013;3:55-64.