2391
Longitudinal changes of cerebral perfusion in patients with moderate traumatic brain injury1Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Charlestown, MA, United States, 2Department of Dermatology, Massachusetts General Hospital, Boston, MA, United States, 3Division of Neuroradiology, Department of Radiology, Massachusetts General Hospital, Boston, MA, United States, 4Department of Emergency Medicine, Massachusetts General Hospital, Boston, MA, United States, 5Department of Cognitive, Linguistic and Psychological Sciences, Brown University, Providence, RI, United States
Synopsis
There is increasing evidence that neurovascular dysregulation contributes to the persistent symptoms in patients with traumatic brain injury (TBI). Damaged microvasculature may disrupt the neurovascular coupling, where the local cerebral blood flow (CBF) no longer matches the metabolic requirements of the tissue. In the present study, we found that there was a diffuse hypoperfusion at thalamus, posterior cingulate gyrus, precuneus and white matter in 2-week follow-up scan relative to the 48-hour initial and 3-month follow-up scans. Low perfusion was sustained in both frontal and parietal cortices in 3-month follow-up scan. Our perfusion findings in these brain regions suggest that hypoperfusion may play a role in the post-traumatic symptoms of moderate TBI.
Introduction
There is increasing evidence that neurovascular dysregulation
contributes to the persistent symptoms in patients with traumatic brain injury
(TBI). The cerebral vasculature was reported to be more sensitive to
blast-induced TBI than other structural elements in animal model 1.
The cerebrovascular pathology varied from mild forms of irregular vessel lumen
and distorted vessel walls to abnormal stricture or stenosis in more severely
affected vessels. These abnormalities in cerebral microvasculature were present
several months after blast exposure, suggesting chronic changes of
microvasculature 1. Damaged microvasculature may disrupt the
neurovascular coupling, where the local cerebral blood flow (CBF) no longer
matches the metabolic requirements of the tissue. That may explain why the
symptoms often reappear or worsen with physical and/or mental exertion after
injury in patients 2-4. To study the baseline cerebral
perfusion changes from acute to chronic stages after TBI, we used pulsed
arterial spin labeling technique to measure CBF in patients with moderate TBI
within 48 hours, 2 weeks and 3 months after the injury. Subjects and Methods
Participants: Thirteen patients with moderate TBI (7 males, 6
females, aged from 21 to 79 years) were included. All of
the patients required hospital admission because of the head
injury. The Glasgow Coma Scale scored above 9 at admission and the
patients also had abnormal imaging findings. Methods: MRI scanning was performed on a 3-Tesla scanner
(Siemens Medical Germany) in the Athinoula A. Martinos Center for Biomedical
Imaging at the Massachusetts General Hospital.
Each patient was scanned within 48 hours (Scan1), 2 weeks (Scan2) and 3
months (Scan3) after the injury. All the
experimental procedures were explained to the patients, and signed informed
consent was obtained prior to participation in the study. Whole brain MRI
datasets were acquired for each patient: 1) standard high-resolution sagittal
images acquired with volumetric T1-weighted 3D-MEMPRAGE (TR=2530ms,
TE=1.69ms/3.55ms/5.41ms/7.27ms, flip angle=7º, FOV=256mm, matrix=256´256, slice
thickness=1mm); 2) pulsed arterial spin labeling sequence (PASL) for the
measurement of baseline perfusion (TR=4000ms, TE=13ms, flip angle=90º,
FOV=220mm, matrix=64×64, thickness=4mm, gap=1mm, inversion time=1800ms, bolus
duration=600ms). Data
analysis: PASL data were imported into the software
Analysis of Functional NeuroImage (AFNI) (National Institute of
Mental Health, http://afni.nimh.nih.gov) for motion correction. The
average CBF image was derived using the PASL equation 6. The
regional change of baseline perfusion was derived based on the parcellation of
T1 image volume using FreeSurfer
(http://surfer.nmr.mgh.harvard.edu). Results
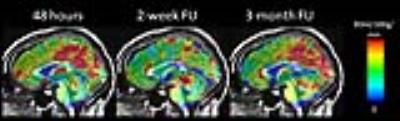
Diffuse hypoperfusion was found in the 2-week follow-up scan.
Lower perfusion was observed specifically at thalamus, posterior cingulate
gyrus, precuneus and white matter in the 2-week follow-up (Scan2) relative to
the 48-hour initial (Scan1) and 3-month follow-up scan (Scan3). Figure 1
shows the baseline perfusion maps of a representative patient. Over 80% of our
patients who completed the 48-hour initial and 2-week follow-up scans show this
changing pattern of baseline perfusion. Figure 2 shows the regional changes
of baseline perfusion across three different time points for all the
patients. While lower perfusion was found in frontal and parietal
cortices in 3-month follow-up scan relative to 48-hour scan, right laterality
of the baseline perfusion increase was observed at the insula and basal
ganglia.Discussion
Thalamic damage has been largely under-investigated in TBI studies, even
though there is converging evidence based on the findings from previous
diffusion tensor imaging and resting state imaging studies. The functions
of the brain regions including thalamus, posterior cingulate, precuneus and
insula documented in the previous literature overlap with the common
post-traumatic symptoms at the chronic stage such as pain, memory retrieval,
sleep problems and difficulty concentrating. Our perfusion findings in
these brain regions suggest that hypoperfusion may play a role in the
post-traumatic symptoms of moderate TBI. Acknowledgements
This research was supported by Congressionally Directed Medical Research Program W81XWH-13-2-0067.References
1. Sosa MA, De Gasperi R, Paulino AJ, et al. Blast overpressure induces shear-related injuries in the brain of rats exposed to a mild traumatic brain injury. Acta Neuropathol Commun 2013;1:51.
2. Strebel S, Lam AM, Matta BF, Newell DW. Impaired cerebral autoregulation after mild brain injury. Surg Neurol 1997;47:128-131.
3. Junger EC, Newell DW, Grant GA, et al. Cerebral autoregulation following minor head injury. J Neurosurg 1997;86:425-432.
4. Golding EM, Robertson CS, Bryan RM, Jr. The consequences of traumatic brain injury on cerebral blood flow and autoregulation: a review. Clin Exp Hypertens 1999;21:299-332.
5. Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996;29:162-173.
6. Wang J, Zhang Y, Wolf RL, Roc AC, Alsop DC, Detre JA. Amplitude-modulated continuous arterial spin-labeling 3.0-T perfusion MR imaging with a single coil: feasibility study. Radiology 2005;235:218-228.
Figures