2389
Proton Magnetic Resonance Spectroscopy of Mild Traumatic Brain Injury in the Military1Center for Clinical Spectroscopy, Brigham and Women's Hospital & Harvard Medical School, Boston, MA, United States, 2U.S. Army Research Institute of Environmental Medicine, Natick, MA, United States
Synopsis
The objective of this ¹H MRS study was to determine the neurochemical profiles of military members with mild Traumatic Brain Injury (mTBI) and compare with age-matched, healthy military controls. Analysis of metabolite concentrations in three brain regions revealed a significant global decrease in total creatine (tCr) in mTBI subjects, as well as elevated GSH in the posterior cingulate and increased Glx in both posterior white matter and anterior cingulate regions, when compared to controls. These results could identify key biomarkers of mTBI and indicate neuroinflammatory changes and impaired brain function or integrity in mTBI.
Introduction
Mild Traumatic Brain Injury (mTBI) is a common incidence in the United States, unfortunately becoming increasingly prevalent in the military. The United States Armed Forces has documented over 350,000 cases of TBI since 20001. Despite its frequency in both military members and civilians, mTBI is still difficult to uniquely diagnose and treat because of the scarcity of imaging tools that can detect it, and especially because it shares many symptoms with Post Traumatic Stress (PTS)2. Magnetic Resonance Spectroscopy (MRS), a non-invasive neuroimaging technique permitting advanced chemical analysis of various brain regions, could be helpful in diagnosing mTBI. The main objective of this 1H-MRS study is to determine the neurochemical profiles of military members suffering mTBI and compare with healthy military controls, in order to identify biomarkers of this condition.Methods
Participants: Military personnel were recruited and consented under local IRB approval to comprise two cohorts: military veterans with mTBI as defined by medical evaluation DSM-IV TR criteria (n=16, age 33.670 ± 7.296, 11 males and 5 females), or veterans without injury (controls; n=18, age 34.889 ± 10.392, 14 males and 4 females). Exclusion criteria included MR contraindications, history of neurological disease, substance abuse, and most neuropsychological disorders except depression and post-traumatic stress which were necessary comorbidities given the scarcity of military veterans with mTBI symptoms only.
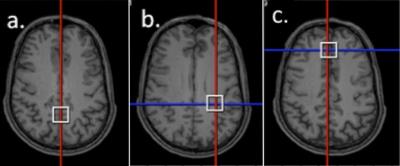
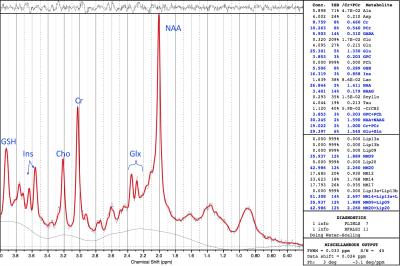
MRS Data Acquisition and Processing: This study was conducted using a Siemens 3T MAGNETOM Skyra and Siemens 3T Trio Tim, both with 32-channel head coils. Three different brain regions were scanned (Figure 1): Posterior Cingulate Gyrus (PCG; 20x20x20mm), Posterior White Matter (PWM; 20x20x20mm), and Anterior Cingulate Gyrus (ACG; 20x20x20mm). Single Voxel 1H MRS data were acquired in each brain region using conventional PRESS, with TE = 30 ms, TR = 2s, bandwidth = 1.2 kHz, 1024 complex data points, water saturation, and 128 averaged acquisitions. Unsuppressed water spectra with the same parameters and 16 averages were also acquired for scaling reference. PRESS data were frequency-corrected and analyzed using LCModel (Figure 2).
Statistical analysis: Processed data were first assessed to identify and exclude metabolites for which Cramer-Rao Lower Bound was greater than 20% indicating unreliable data. Remaining metabolites included Glu, Glx, GPC+PCh, GSH, mI, NAA+NAAG, and total creatine (tCr). The ratios of metabolite concentrations to tCr were then compared between cohorts using Welch’s t-tests (GraphPad Prism 7). This correction was applied to adjust for the difference in cohort sample size and unequal variance between cohorts.
Results
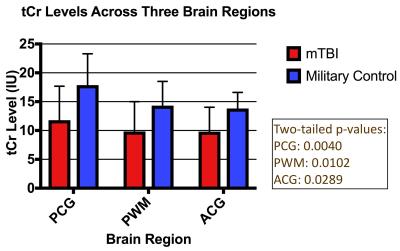
Comparative analysis of metabolite concentrations in military mTBI subjects and healthy military controls revealed global decreases in tCr in mTBI subjects (Figure 3). Additionally, mTBI subjects showed elevated GSH levels in PCG (p = 0.0397) and increased Glx in both PWM (p = 0.0023) and ACG (p = 0.0289). P-values are two-tailed and show significant differences between the mTBI and control cohorts (p < 0.05).Discussion
Creatine plays an essential role in maintaining brain energy homeostasis by shuttling high-energy phosphate bonds between subcellular compartments.3 Reductions in tCr could be indicative of impaired brain function or integrity3 in mTBI patients.
GSH is an anti-oxidant that removes or reduces damaging reactive oxygen species such as free radicals and peroxides, and is therefore directly involved in oxidative stress and neuroinflammation. Increased GSH may be a mechanism for early compensatory or neuroprotective response to oxidative stress brought on by neuroinflammation4. While results of this study show GSH increases only in the PCG of mTBI subjects, elevated GSH has also been observed in the ACG of PTS subjects5. This suggests that the location of neurometabolic changes within the brain might be key in uniquely identifying mTBI and distinguishing from PTS.
Glx is the combined signal measurement of glutamate and glutamine in 1H-MRS. Glutamate is the most abundant excitatory neurotransmitter, while glutamine primarily serves as a non-neuroactive intermediate in the recycling of amino acid neurotransmitters.3 These results showing elevated Glx in the PWM and ACG are consistent with previous findings recording Glx increases of ~33% in professional athletes with repetitive brain trauma6, and are predictive of poor outcome in severe TBI7.
This is, to our knowledge, the first study of military mTBI at 3T, with only one other study at 7T8 in which PTS status did not impact results. Additional MRS research elucidating differences between mTBI and PTS would bolster the statistically significant results from this study and further clarify measures of mTBI.
Conclusion
The powerful ability of MRS to quantify small neurometabolic differences in military veterans with mTBI will allow for clearer characterization of mTBI and the advancement of strategies for its diagnosis and treatment.Acknowledgements
This research was conducted with financial support from the Department of Defense Congressional Directed Medical Research Program (WX81-XWH-10-1-0835) and statistical support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102). Special thanks to Drs. Randall Scheibel, Maya Troyanskaya, Lisa Wilde, and Brian Taylor at Baylor College of Medicine, Houston, TX, who contributed data to this study by recruiting and scanning six of the subjects for the mTBI cohort, under the same protocol described in Methods. Disclaimer: The views expressed in this abstract are those of the authors and do not reflect the official policy of the Department of Army, Department of Defense, or the U.S. Government.References
1. Defense and Veterans Brain Injury Center, Department of Defense. DoD TBI World Numbers since 2000, PDF. 12 August 2016. Web Access: 8 November 2016. http://dvbic.dcoe.mil/files/tbi-numbers/DoD-TBI-Worldwide-Totals_2000-2016_Q1-Q2_Aug-12-2016_v1.0_508_2016-09-20.pdf
2. Lin AP, Koerte IK, Willems A, Muehlmann M, Hufschmidt J, Coleman MJ, Green I, Liao H, Tate DF, Wilde EA, Pasternak O, Bouix S, Rathi Y, Bigler ED, Stern RA, Shenton ME. A Review of Neuroimaging Findings in Repetitive Brain Trauma. Brain Pathology. 2015; 25(3):318-49.
3. Maddock, RJ and Buonocore, MH MR Spectroscopic Studies of the Brain in Psychiatric Disorders. Current Topics in Behavioral Neurosciences. 2012; 11:199-251.
4. Duffy, SL et al. Glutathione relates to neuropsychological functioning in mild cognitive impairment. Alzheimer's & Dementia. 2014; 10(1):67-75
5. Michels, L et al. Prefrontal GABA and glutathione imbalance in posttraumatic stress disorder: Preliminary findings. Psychiatry Research: Neuroimaging. 2014; 224(3):288-95
6. Lin AP et al. Changes in the neurochemistry of athletes with repetitive brain trauma: preliminary results using localized correlated spectroscopy. Alzheimer's Research & Therapy. 2015; 7:13
7. Chamoun R, Suki D, Gopinath SP, Goodman JC, Robertson C. Role of extracellular glutamate measured by cerebral microdialysis in severe traumatic brain injury. J Neurosurg. 2010; 113:564–70.
8. Hetherington H, Hamid H, Kulas J, et al. MRSI of the Medial Temporal Lobe at 7T in Explosive Blast Mild Traumatic Brain Injury. Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2014; 71(4):1358-1367.
Figures