2388
MRI based early monitoring and quantitative assessment of macrophages infiltration after experimental traumatic brain injury in mice1NMR Research Centre, Institute of Nuclear Medicine and Allied Sciences, Delhi, India, Delhi, India, 2Division of Stem Cell and Gene Therapy Research, Institute of Nuclear Medicine and Allied Sciences, Delhi, India, Delhi, India
Synopsis
The inflammatory response following traumatic brain injury (TBI) is regulated by phagocytic cells, comprising resident microglia and infiltrating macrophages. The present study was to monitor the early effect of monocytes/phagocytic accumulation and further to explore its kinetics in TBI mice. Localized macrophage population was monitored using USPIO nanoparticles enhanced in vivo serial magnetic resonance imaging (MRI). Flow cytometry based gating study was performed to discriminate between resident microglia (Ly6GˉˉCD11b+CD45low) and infiltrating macrophages (Ly6GˉCD11b+CD45high). Imaging and flow cytometric analysis revealed that maximum macrophage infiltration occurs between 66-72 h post injury (42-48 h post administration of USPIO) at the site of inflammation.
Introduction
Traumatic brain injury (TBI) is a significant health problem in victims of sports, accidents and military combatants. There are no clinically approved drugs or biologics available till now1,2. Recent research suggests that infiltrating macrophages may play a pivotal role at the interface between early detrimental and delayed beneficial effects of inflammation3. Therefore, inhibition of infiltrating macrophages is considered as an attractive therapeutic strategy. Several published reports have shown a time frame of 1-7 days for macrophage accumulation at the neuroinflammatory site using MRI and immunohistochemistry, but no one has so far precisely established the specific time window. Here we also describe a flow cytometry-based method to differentiate resident microglia and infiltrating macrophages after gating out activated neutrophils in a murine model of closed head TBI.Aim and Objectives
1. To investigate the feasibility of phagocyte/macrophage labeling and its serial in vivo monitoring through MRI after experimental TBI.
2. To explore the kinetics of phagocytic cells at the injury site and the discrimination between resident microglia and infiltrating macrophages by MRI and flow cytometry, respectively.
Materials and Methods
All experimental procedures were conducted under the approval of the animal ethics committee of the Institute. Out of 52 mice taken for study, 32 mice were randomly selected for MRI and other 20 mice for flow cytometry analysis. Mice selected for MRI were further subdivided into four groups. Mice in group-1 (n=8) were injected with 0.9% NaCl after TBI, group-2 mice (n=8) were injected with USPIO without any injury, group-3 & 4 mice (n=8 each) were injected with USPIO after TBI. The USPIO were administered (3.0 mg Fe/kg body weight) to each mouse after 24 h of injury.
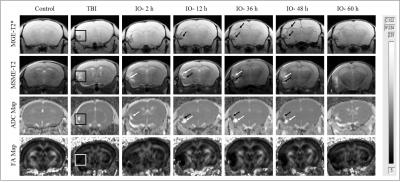
All MRI experiments were carried out on a 7T horizontal bore animal MRI scanner (Bruker Biospec USR 70/30, AVANCE III). T2 maps were acquired using MSME sequence (TR= 3500 ms, TE = 13-208 ms with echo length of 13 ms, Flip angle = 180o). T2* maps were acquired using MGE sequence (TR = 1500 ms, 12 echos TE/echo spacing= 4.50-65.45 ms/5.54 ms, matrix size = 256/192, Flip Angle = 30o). Diffusion-weighted images were acquired using DTI sequence (TR/TE = 3800/31 ms, number of diffusion encoding directions = 46, b value = 670 sec/mm2, matrix size = 128x128). ROI’s (area = 0.0211 cm2) were drawn at the site of injury in consecutive three slices with three ROI’s per slice. T2* relaxation times (msec) were calculated by fitting the signal decay curve of multi-echo data using single exponential curve fitting in association with ‘absolute bias’ correction.
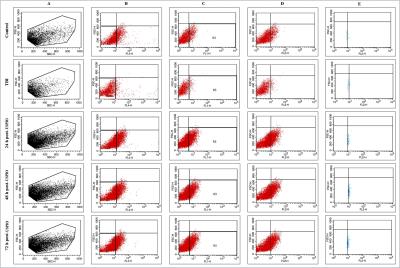
The brain was removed and the identical injured area (2.5x2.5x2.5 mm3) was dissected out with the help of MR images. Single cell suspension from brain tissue was prepared by using neural tissue dissociation kit and samples were incubated with Ly6G-APC, CD45-PE and CD11b-FITC. Flow cytometric data acquisition were carried out having 530/30, 585/42, 670LP and 661/16 nm lasers.
Results and Discussion
There was no significant change in signal intensity at different time intervals in control mice (group 1 & 2). ADC map revealed an increase in signal intensity after induction of injury, which is due to accumulation of water content after neuroinflammation (Fig.1). T2* images and FA maps showed a decrease in signal intensity at later time points (post-TBI) after administration of USPIO. MSME-T2 images in Figure-1 demonstrated a combination of hyper and a hypointense region, which indicates both inflammatory water content (white) and accumulation of iron content (black). No significant difference in T2* time was observed in group-1 & 2. Whereas an immense significant decrease in transverse relaxation time was calculated in group-3 (36 h, P: 0.0087; 48 h, P: 0.00002 and 60 h, P: 0.0014) and group-4 (42 h, P: 0.0001; 54 h, P: 0.0016 and 72 h, P: 0.0002) as compared to TBI.
Flow cytometry revealed that the numbers of microglia/macrophages were insignificant in injured brain (15.5 ± 5.5%) as compared to control (10.7 ± 3.1%). The percentage of macrophage cells was increased significantly at different time points following post-USPIO administration (24 h, 61.4 ± 6.6%; 48 h, 77.2 ± 5.6%; 72 h, 71.2 ± 8.8%) (Fig.2). Approximately 7.2 and 6.6 fold increase in infiltrating population of macrophage was observed at 48 h and 72 h post-USPIO administration (72 h and 96 h post-injury)
Conclusion
Imaging and flow cytometry based quantitative analysis revealed that maximum infiltration of macrophages was observed at 48 h post-USPIO injection (72 h post injury) after experimental TBI.Acknowledgements
The project (INM-311) was financially supported by DRDO, Ministry of Defence, Govt. of India and fellowship supported from ICMR is gratefully acknowledged.References
1. Jain K K. Neuroprotection in traumatic brain injury. Drug Discov. Today 2008; 13: 1082-1089.
2. Mishra S K, Rana P, Khushu S, Gangenahalli G. Therapeutic prospective of infused allogenic cultured MSCs in traumatic brain injury mice: A longitudinal proton magnetic resonance spectroscopy assessment. Stem Cells Transl Med. 2016; 5: 1-16.
3. Gensel J C and Zhang B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 2015; 14: 01752-01761.
Figures