2387
Quality control for human brain advanced diffusion imaging for assessment of concussion1Neurology, Washington University School of Medicine in St. Louis, St. Louis, MO, United States
Synopsis
Most concussive brain injury is not readily detected in conventional MRI or standard DTI, requiring advanced imaging methodology. However, reliability of any newly developed imaging technology should be tested prior to starting full scale studies. Here we present a protocol which will enable inter-lab comparison of any advanced diffusion imaging technology with objective quantitative analysis. High spatial resolution diffusion imaging with optimized multiband pulse sequence performed on subjects twice at 1.25 mm isotropic voxel size. Post image processing including outlier exclusion and distortion correction was optimized. Brain parcellation based quantitative analyses was used to provide objective measures of reproducibility.
Purpose
In the presented study, we propose a protocol to examine the reproducibility of advanced human brain diffusion imaging. Recently new MR hardware and software have been developed like high power main magnets (3 – 7 Tesla) and high power imaging gradients (80 – 100 mT/m), multi-channel RF coils, multi-band pulse sequences, etc. These advanced technologies enable collection of high spatial and angular resolution human brain diffusion imaging data. Consequently, the human brain diffusion images reflect fine micro structure which was not seen in vivo previously (1-3). The advanced diffusion imaging would be highly beneficial for concussive traumatic brain injury which injury is not readily seen in conventional MRI or standard diffusion tensor imaging. Most traumatic brain injury occurs in inferior orbitofrontal and temporal pole regions where MR image artifact is most significant. Thus, prior to starting full scale studies using advanced methodologies, their reproducibility should be tested, especially in challenging anatomical regions.Methods
All MR data were collected using Siemens Prisma scanner, 3 T and 80 mT/m gradient, with 32 channel coil. T2 weighted images (T2W) at 0.8 mm isotropic voxel resolution were collected using rapid acquisition with relaxation enhancement (RARE) with RARE factor 8, repetition time (TR, 6 s), and echo time (TE, 560 ms). Diffusion images were acquired using 2 D echo planar imaging multi-band sequence (4) with 6/8 Fourier, 6200 ms TR, and 110 ms TE. Diffusion images at 1.25 mm isotropic voxel were acquired with opposite phase encoding direction, anterior-posterior and posterior-anterior. 30 diffusion weighted images with b-value 1000 s/mm2 were acquired including 5 b0 images. Four normal controls and three concussive TBI patients underwent two MR measurements 7 – 21 days apart. After data collection, all images were normalized in Siemens platform. The normalized magnitude diffusion image data underwent post image processing and quantitative analyses, which includes 1) image format conversion using MRICRON program (http://people.cas.sc.edu/rorden/mricron/index.html), 2) outlier exclusion (see Figure 2 and 3), 3) distortion correction using TORTOISE and DR-BUDDI (5) (https://science.nichd.nih.gov/confluence/display/nihpd/TORTOISE), 4) masking and diffusion metrics calculation using DIFF_CALC in TORTOISE, and 5) parcellation and quantification analysis using DTI_STUDIO (https://www.mristudio.org/). Bland-Altman plots were used to examine reproducibility of the whole diffusion imaging protocol.Results
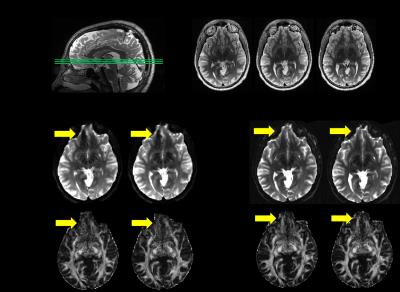
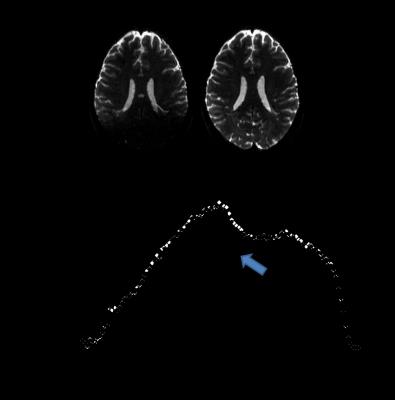
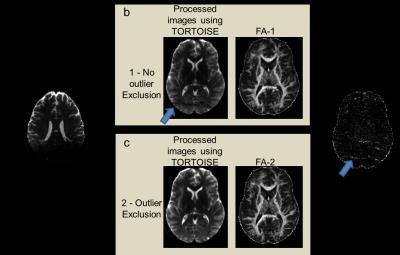
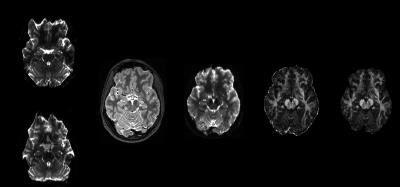
Figure 1 shows T2W, b0, and fractional anisotropy (FA) for the orbito frontal region which is well known as highly vulnerable to TBI (6). The multi-band factor 4 showed significant signal loss where multi-band factor 2 preserved the tissue signal, allowing FA calculations. Figures 2 and 3 show motion related MR signal drop in diffusion images, which is typical in single shot echo planar images. The signal loss type artifact was readily detected by measuring the image intensity, Fig. 2. These outliers were removed prior to post image processing in TORTOISE. Without outlier exclusion, the distortions propagate throughout image volumes, Fig. 3. Diffusion image distortion is almost inevitable in EPI sequences. There are readily applicable distortion correction open source approaches such as EDDY in FSL (7) and DR-BUDDI in TORTOISE (5). Both require diffusion images with opposite phase encoding directions. Figure 4 a – d show that distortion at orbital frontal region was successfully corrected using DR-BUDDI. After distortion correction, diffusion metrics were calculated after masking non-brain regions. Poor brain masking may leave speckles, which ruin accuracy for quantitative analysis, Fig. 4e. The masking quality was regulated using DIFF_CALC in TORTOISE, which also utilizes the brain extraction tool in FSL (8). The masking result was controlled by mainly three parameters, noise mean, fractional intensity threshold, and erosion factor. In the presented study, we used 4 noise mean, 0.2 fractional intensity factor, and 3 erosion factor. Minor manual masking was required only for temporal pole region. After brain masking and diffusion metrics calculation, parcellation based quantitative analysis was done using DTI_STUDIO. Figure 5 shows the Bland-Altman plot of two FA measures for whole brain from same subject. All quantified measures were located within 95% confidence limits showing high reproducibility of the employed 1.25 mm isotropic human brain diffusion imaging protocol.Discussion and Conclusion
We have presented a protocol designed for assessment of concussive TBI which includes optimizing pulse sequence, outlier exclusion, distortion correction, brain masking, and quantitative measure of reproducibility. Most advanced diffusion imaging methodology involving high spatial resolution and increased b-value (diffusion attenuation) might result in reduced MR image intensity, which might make the obtained image either less robust or vulnerable to artifact. The suggested protocol will provides objective measure of reproducibility for any advanced diffusion imaging methodologies.Acknowledgements
This study was supported by. NIH U01 NS086659-01, the US Department of Defense, and HealthSouthReferences
1. Fan Q, Witzel T, Nummenmaa A, Van Dijk KR, Van Horn JD, Drews MK, Somerville LH, Sheridan MA, Santillana RM, Snyder J, Hedden T, Shaw EE, Hollinshead MO, Renvall V, Zanzonico R, Keil B, Cauley S, Polimeni JR, Tisdall D, Buckner RL, Wedeen VJ, Wald LL, Toga AW, Rosen BR. MGH-USC Human Connectome Project datasets with ultra-high b-value diffusion MRI. NeuroImage 2016;124(Pt B):1108-1114.
2. Hodge MR, Horton W, Brown T, Herrick R, Olsen T, Hileman ME, McKay M, Archie KA, Cler E, Harms MP, Burgess GC, Glasser MF, Elam JS, Curtiss SW, Barch DM, Oostenveld R, Larson-Prior LJ, Ugurbil K, Van Essen DC, Marcus DS. ConnectomeDB--Sharing human brain connectivity data. NeuroImage 2016;124(Pt B):1102-1107.
3. Vu AT, Auerbach E, Lenglet C, Moeller S, Sotiropoulos SN, Jbabdi S, Andersson J, Yacoub E, Ugurbil K. High resolution whole brain diffusion imaging at 7T for the Human Connectome Project. NeuroImage 2015;122:318-331.
4. Todd N, Moeller S, Auerbach EJ, Yacoub E, Flandin G, Weiskopf N. Evaluation of 2D multiband EPI imaging for high-resolution, whole-brain, task-based fMRI studies at 3T: Sensitivity and slice leakage artifacts. NeuroImage 2016;124(Pt A):32-42.
5. Irfanoglu MO, Modi P, Nayak A, Hutchinson EB, Sarlls J, Pierpaoli C. DR-BUDDI (Diffeomorphic Registration for Blip-Up blip-Down Diffusion Imaging) method for correcting echo planar imaging distortions. NeuroImage 2015;106:284-299.
6. Gurdjian ES, Gurdjian ES. Cerebral Contusions: Re-evaluation of the mechanism of their development. Journal of Trauma and Acute Care Surgery 1976;16(1):35-51.
7. Andersson JL, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage 2016;125:1063-1078.
8. Smith SM. Fast robust automated brain extraction. Human brain mapping 2002;17(3):143-155.
Figures