2374
MRI of longterm changes in vascularization and functional connectivity in a mouse model of vascular cognitive impairment1Department of Experimental Neurology, Center for Stroke Research Berlin, and NeuroCure, Charité University Medicine Berlin, Berlin, Germany, 2Charité Core Facility 7T experimental MRIs, Charité University Medicine Berlin, Berlin, Germany, 3Department of Neurology, Berlin Center for Advanced Neuroimaging, NeuroCure Clinical Research Center Neuroimmunology, and Berlin School of Mind and Brain, Charité University Medicine Berlin and Humboldt University Berlin, Berlin, Germany, 4School of Life Sciences, University of Nottingham, Nottingham, United Kingdom
Synopsis
Chronic mouse brain hypoperfusion produces white matter damage; a feature of vascular cognitive impairment. Despite growing interest in this model, we have struggled to observe a strong phenotype. The present study aimed to improve the phenotype through extended hypoperfusion (6m). We examined the effect on various MR biomarkers including functional connectivity and vascular remodeling. We found massive structural changes including arterial neovessels, small subcortical strokes, and microbleeds. Animals showed behavioral deficits accompanied by changes in resting state MRI signals of the cingulate cortex, which is functionally connected to regions related to behavior (hippocampus) and emotion (amygdala).
Purpose
Chronic hypoperfusion in mice by implantation of constricting microcoils around both carotid arteries has been shown to mimic aspects of vascular cognitive impairment (VCI)1. Despite growing interest in the model we have failed to reproduce a strong behavioral phenotype2. To address this, we have previously reduced microcoil diameter and could show changes in MR angiograms of the Circle of Willis and in the structural connectome assessed by network analysis of fiber tracts reconstructed from diffusion tensor imaging data six weeks after surgery3. Despite the more severe hypoperfusion procedure, the phenotype remained mild. The goal of the present study was to find new neuroimaging biomarkers of the disease and to examine whether the phenotype could further be improved by increasing the survival time. We assessed changes in various MR and behavioral biomarkers including vascularization and functional connectivity in mice surviving up to 6 months after surgery.Methods
6 wk
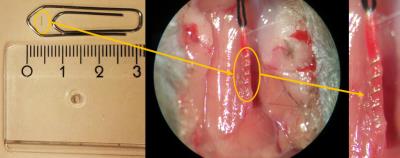
old C57/Black6 mice were randomized and underwent implantation of nonmagnetic
surgical-grade steel microcoils (160 µm, n=10, Shannon, Limerick, Ireland) or
the sham procedure (500 µm, n=10) with 24 h in between left and right
implantation to improve survival (Fig. 1). Experimenters were blinded to the
condition of animals. Using various room temperature probeheads and a 7T system
(Bruker BioSpin, Ettlingen, Germany) animals underwent serial T2-weighted MRI
(RARE, 32 contiguous 0.5 mm thick slices, FOV=(25.6 mm)2, Matrix MTX=2562, TR/TE=4200/36 ms, Number of
Averages NA=4, TA=6:43 min), cerebral blood flow (CBF) measurements (FAIR-RARE,
1 mm thick slice, FOV=(25.6 mm)2, MTX=1282, 16 TIs=35-1600 ms,
TR/recovery time/TE=12000/10000/35.9 ms, TA=12 min), angiography (3D-TOF,
FOV=20x20x16 mm3, MTX=200x200x160, TR/TE=15/2.5 ms, FA=20°, TA=6 min).
Susceptibility weighted imaging (SWI-FLASH, 20 contiguous 0.5 mm thick slices,
FOV=(19.2 mm)2, MTX=2562, TR/TE=700/18 ms), MR spectroscopy (STEAM,
TR/TE/mixing time: 2500 ms/3 ms/10 ms, NA=256, TA=10:40 min, LCModel analysis)
and rsMRI (single-shot GE-EPI, 12 contiguous 0.75 mm thick slices, FOV=(19.2
mm)2,
MTX=128x80, TR/TE=1000/10 ms, TA=5 min, 1-1.25% isoflurane in 30/70 O2/N2O)
were carried out with a Cryoprobe. RsMRI data were registered to the MRMNeAt
atlas and analyzed with group independent component analysis (ICA) using FSL's
MELODIC tool (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/) fixed to 90 ICA
components. Animals underwent behavioral testing before processing the brains
for RNA sequencing and histology.
Results&Discussion
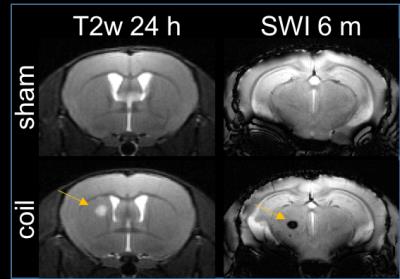
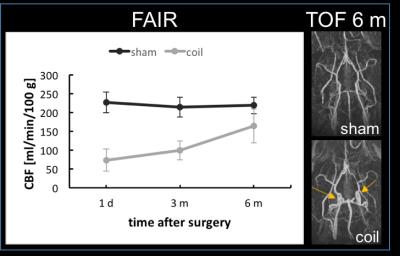
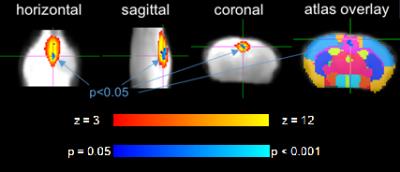
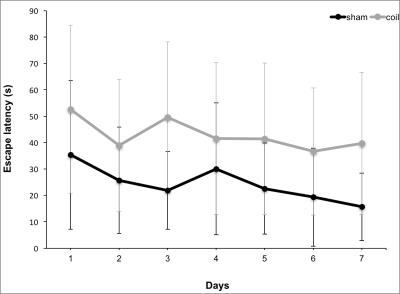
4/10 animals had small subcortical lesions on T2-weighted images 24 h post-surgery. 4/10 (2/4 with T2 lesion) died during the first 3 months. 2/6 displayed microbleeds on SWI at 6 m, which was never observed in shams (Fig. 2). CBF was initially reduced by ~60% and improved over 6 months, which was accompanied by massive vascular remodeling in the posterior Circle of Willis (Fig. 3). Most changes in MRS metabolite concentrations were related to age and not surgery but full statistical analysis is pending. Dual regression of rsMRI at 6 months showed significant differences between coil and sham animals in an ICA component in the cingulate cortex (Fig. 4). This component has been shown to be highly connected to regions important for behavior and emotion, e.g. hippocampus and amygdala4. Coil animals exhibited a learning impairment in the watermaze at 6 m (Fig. 5) but detailed behavioral and ex vivo analyses are pending.
Conclusion
Using nonmagnetic microcoils and a FAIR protocol, hypoperfusion and compensatory processes can robustly be monitored in VCI mice. We found a stronger phenotype beyond the previously chosen time window of 6 weeks. Under these conditions, the mouse model reflects additional aspects of VCI in patients not previously reported including subcortical strokes and microbleeds and, for the first time, changes in functional connectivity. The quantitative MRI methods developed herein can be used to investigate interventions that target VCI outcome, e.g. in transgenic or pharmaceutic approaches.Acknowledgements
Work was supported by the Federal Ministry of Education and Research (01EO0801, Center for Stroke Research Berlin) and Deutsche Forschungsgemeinschaft (Excellence Cluster NeuroCure).References
1. Shibata et al. Stroke(2004);35:2598–603.
2. Füchtemeier et al. JCBFM(2015); 35: 476–84.
3. Boehm-Sturm et al. EMIM(2016);abstractID#209.
4. Mechling et al. Neuroimage(2014); 96: 203–215.
Figures