2371
Association between whole brain functional connectivity and cortical thickness throughout healthy aging1InBrain Lab, Department of Physics, Faculty of Philosophy, Sciences and Letters of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil
Synopsis
The aging process entails morphological and functional alterations in the human brain. Using magnetic resonance imaging data of 130 subjects aged between 18 and 81 years from a publicly available dataset we obtained whole brain cortical thickness estimates and resting state connectivity to study how healthy aging affects these. Additionally, we studied the relationship between cortical thickness and functional connectivity. A heterogeneous thinning profile was observed and also a dominance in increases in connectivity, with few decreases. Connectivity correlates with thickness with temporal and occipital seed ROIs. These results might help to understand how connectivity and thickness relate in neuropathologies.
Introduction
Worldwide the elder population is growing 1. Healthy aging entails several changes in both anatomy 2,3 and function 5-8 in the cerebral grey matter, and these alterations partially correlate with observed cognitive decline. Both structural and functional changes can be observed through MRI and fMRI, respectively.
Previous studies showed how aging affects cortical thickness 2-4 where a heterogeneous thinning pattern is observed, usually with greater decline in frontal and parietal regions. Whole brain connectivity alterations solely due to aging has been previously studied as well 5-8. In general, functional alterations are observed in the motor cortex 9 and around the areas associated with the default mode network 6, reflecting the decline in motor control and the dedifferentiation of the brain, respectively. Studies combining functional connectivity and grey matter integrity information have been performed as well 10, but these are usually focused on specific brain regions.
The present study focuses on the whole brain association between cortical thickness and functional connectivity during healthy aging.
Methods
Anatomical and functional 3.0 T MR images from 207 subjects were obtained in the database created and maintained by the NKI 11. Exclusion criteria included age less than 18 years old, left-handed individuals or visible motion artifact in the anatomical scan. 130 subjects were retained.
MP-RAGE data was processed in Freesurfer v5.3.0 12 through a default pipeline, resulting in a parcellation and cortical thickness estimates according to a gyral-sulcal atlas 13.
In CONN v.16.b 14 the resting state functional scans were pre-processed with a default subject-space pipeline using the Freesurfer parcellation. The 6-directional movement parameters and scrubbing were included as covariates in the connectivity calculation.
Linear regression was performed between the cortical thickness for each ROI across subjects and their respective age, controlling for sex and an interaction term between sex and age. Also, for each of the unique 10878 connectivities a GLM was analogously performed.
To study the interaction between thickness and connectivity during aging, for each ROI, multilevel analysis was performed in R. In the first level, for each subject, the difference in thickness between a target ROI and the seed ROI was regressed out of the Z-transformed functional connectivity between both. At the second level, the angular coefficients obtained for each subject in the first level were used as dependent variables in a regression including age, sex, and their interaction as independent variables. Every coefficient of every analysis was compared to a zero point-null hypotheses. Correcting for multiple comparison, a p-FDR 15 less than 0.05 was defined as significant
Results
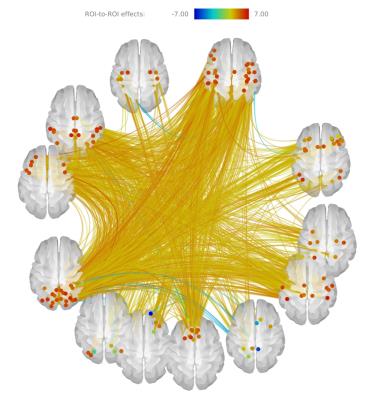
Aging strengthens functional connections across the whole brain. Exceptions include connectivities along the medial surfaces among areas such as the precuneus, posterior cingulate and rectus of both hemispheres, as in Figure 1.
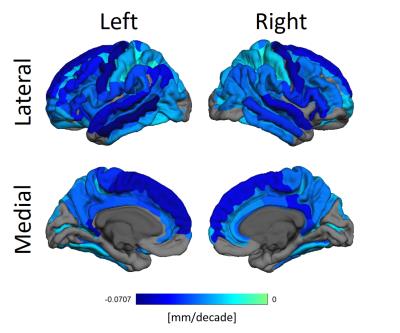
Figure 2 shows how thickness decreases heterogeneously. Areas such as the pre-central, superior frontal, middle temporal and the ventral posterior cingulate gyri are specially affected.
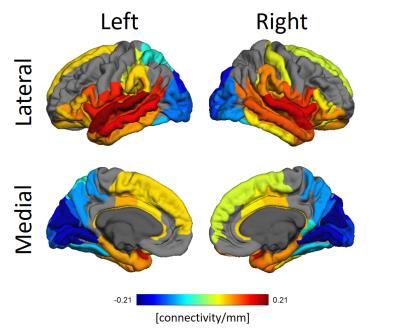
In Figure 3 the relationship between functional connectivity from a seed ROI to all other ROIs as targets and the difference in thickness between target and seed ROIs discounting effects of age and sex is shown.
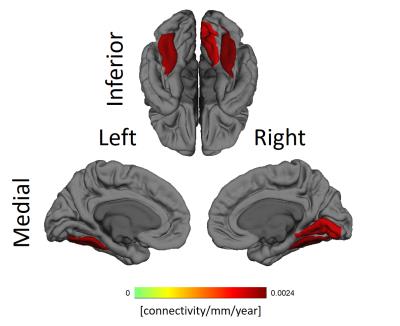
The fusiform gyri and the right lingual gyrus showed significant increase with aging in the dependence between functional connectivity and cortical thickness, as can be seen in Figure 4.
Discussion
A generalized increase in connectivity was previously reported in the literature 6,16,17. Around six percent of the connections present significant alterations due to age. Taking into account the cognitive decline usually observed in elders the increase in connectivity can be seen as evidence of compensation mechanisms operating due to aging, analogously to what is observed in several neurological diseases. Connectivity decreases are also consistent with previous works.
Cortical thinning due to aging is well established, and our results corroborate that notion. We, however, did not find any significant cortical thickening in the cortex. This can be due to methodological aspects of our work.
The whole brain association between cortical thickness and functional connectivity of ROIs reveal self-consistencies regarding both properties. Occipital lobes are usually thinner while temporal lobes are thicker. Figure 3 clearly shows those lobes have, respectively, diminished and increased connectivity the thicker the target ROIs. The significant alterations in the gyri along the occipitotemporal cortex suggest alterations in this area known for visual perceptual functions.
Conclusion
We have evidence connectivity and thickness present a near constant association throughout healthy aging. The findings in our study could help elucidate the association between connectivity and thickness alterations, with possible applications in the study of how neuropathologies marked by either or both phenomena develop.Acknowledgements
The authors would like to acknowledge CNPq, for the grant provided.References
1. Centers for Disease Control and Prevention. Trends in aging-United States and worldwide. MMWR. Morbidity and mortality weekly report, 52(6), 101.
2. Salat DH, Buckner RL, Snyder AZ, et al. Thinning of the cerebral cortex in aging. Cerebral cortex. 2004;14(7):721-730.
3. Thambisetty M, Wan J, Carass A, et al. Longitudinal changes in cortical thickness associated with normal aging. Neuroimage. 2010;52(4):1215-1223.
4. Sowell ER, Peterson BS, Kan E, et al. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cerebral cortex. 2007;17(7):1550-1560.
5. Ferreira LK, Busatto GF. Resting-state functional connectivity in normal brain aging. Neuroscience & Biobehavioral Reviews. 2013;37(3):384-400.
6. Ferreira LK, Regina ACB, Kovacevic N, et al. Aging effects on whole-brain functional connectivity in adults free of cognitive and psychiatric disorders. CerebCortex. 2015;26(9):3851-3865.
7. Sala-Llonch R, Bartrés-Faz D, Junqué C. Reorganization of brain networks in aging: a review of functional connectivity studies. Frontiers in psychology. 2015; 6:663.
8. Wu T, Zang Y, Wang L, et al. Normal aging decreases regional homogeneity of the motor areas in the resting state. Neuroscience letters. 2007;423(3):189-193.
9. Seidler R, Erdeniz B, Koppelmans V, et al. Associations between age, motor function, and resting state sensorimotor network connectivity in healthy older adults. NeuroImage. 2015;108:47-59.
10. Marstaller L, Williams M, Rich A, et al. Aging and large-scale functional networks: White matter integrity, gray matter volume, and functional connectivity in the resting state. Neuroscience. 2015;290:369-378.
11. Nooner KB, Colcombe SJ, Tobe RH, et al. The NKI-Rockland sample: a model for accelerating the pace of discovery science in psychiatry. Front. Neurosci. 2012;6:152.
12. Fischl B. FreeSurfer. Neuroimage. 2012;62(2):774-781.
13. Fischl B, van der Kouwe A, Destrieux C, et al. Automatically parcellating the human cerebral cortex. Cerebral cortex. 2004;14(1):11-22.
14. Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain connectivity. 2012;2(3):125-141.
15. Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Annals of statistics. 2001;1165-1188.
16. Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Annals of statistics. 2001;1165-1188.
17. Meier TB, Desphande AS, Vergun S, et al. Support vector machine classification and characterization of age-related reorganization of functional brain networks. Neuroimage. 2012, 60(1), 601-613.
Figures