2365
Synergistic Effect of β-Amyloid and Microvascular Abnormality on Longitudinal Cognitive Decline in Elderly Subjects at Risk for Alzheimer’s Disease (AD)1Neurosection, Div. of MRI Research, Dept. of Radiology, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States, 3Institute for Biomedical Engineering, University of Zürich and ETH Zürich, Zürich, Switzerland, 4Division of Psychiatry Research and Psychogeriatric Medicine, University of Zürich, Zürich, Switzerland, 5Division of Nuclear Medicine, University of Zürich, Zürich, Switzerland, 6Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, MD, United States
Synopsis
Progressive impairment in multiple cognitive domains is a clinical hallmark of Alzheimer’s Disease (AD), which is the most frequent cause for dementia in the elderly and is neuropathologically characterized by both cerebral β-amyloid (Aβ) accumulation and microvascular abnormalities. Here, we report significant co-localization of regions with microvascular abnormalities measured by arteriolar-cerebral-blood-volume (CBVa) MRI and Aβ accumulation measured by PiB-PET in elderly subjects at-risk for AD. Multiple regression analysis suggested that CBVa and Aβ may have a synergistic effect on longitudinal cognitive decline in these subjects . Both variables may need to be considered for secondary prevention trials in such populations.
PURPOSE:
Short-term cognitive decline is often used as an outcome measure in secondary prevention trials in clinically normal (CN) populations at high risk for Alzheimer’s Disease (AD). It is therefore crucial to investigate the relationship between neuroimaging biomarkers for AD and longitudinal cognitive decline in asymptomatic at-risk populations. PiB-PET imaging can be employed to estimate cortical β-amyloid(Aβ)-plaque-load, which serves as an indicator of Aβ accumulation that has been linked to AD as a key pathologic feature. Moreover, Aβ accumulation is also found in about one-third of elderly CN individuals1. However, some subjects with substantial Aβ accumulation show much less cognitive decline than the others, which implies influence from additional factors that may aggrevate cognitive impairment. Cerebrovascular dysfunction has been associated with aging, mild cognitive impairment (MCI) and AD2. Pial arteries and arterioles are the blood vessels that are most responsive to changes in metabolism and are affected before capillaries and venous vessels in aging.3 We have recently reported abnormality in the combined cerebral-blood-volume of these vessels (CBVa) in several brain regions in elderly subjects at risk for AD, and its association with APOE-e4 carrier-status (a major genetic risk factor for sporadic AD)4. Our purpose here is to investigate the association between Aβ and CBVa markers, and whether these factors act additively or synergistically on short-term cognitive decline in a group of elderly subjects at risk for AD.METHODS:
Aging of the brain is considered the major risk factor for AD. Although MCI can be caused by other pathologies, subjects with MCI are at increased risk of developing AD dementia1. Therefore, healthy elderly individuals and subjects with MCI can be used to investigate prodromal AD biomarkers. Participants and cognitive tests: Eighteen subjects with MCI (11male, 7female; 75.0±7.2yr) and twenty-two healthy elderly controls (14male, 8female; 72.0±5.3yr) were scanned. Twelve subjects carried one APOE-e4-allele, while two subjects carried two APOE-e4-alleles. Each participant had two visits approximately 2 years apart (730±277 days). All participants received neuropsychiatric examination (four cognitive tests to assess language, working memory, executive function, and episodic memory, respectively) and were screened for cognitive impairment. Subjects were categorized either as cognitively normal or MCI according to established criteria5. Cognitive measures were z-score transformed and averaged to generate one global score for each subject. PiB-PET based estimation was used to measure cortical Aβ-plaque-load6. Cortical PiB retention scores were determined by calculating a composite score using merged cortical PiB-PET intensity values7. MRI: 7T Philips scanner, 32-channel head coil. Anatomical images were acquired with MP2RAGE (voxel=0.75mm isotropic). CBVa was measured using 3D iVASO MRI with whole brain coverage4. SPM8, AIR and in-house code (Matlab6.0, Mathworks) were used for analysis8. Statistics: Two-sample t-tests were performed to examine group difference in CBVa in the whole brain on a voxel-by-voxel basis. Age, sex, education, cortical thickness and residual motion parameters (after motion correction) were all accounted for as covariates. Partial correlations were calculated with age, sex and education as covariates. Multiple comparisons were corrected with the false-discovery rate (adjusted P < 0.05). Multiple regression was carried out to test the potential synergistic effects from CBVa and β-Amyloid (reflected in the β3 term) on longitudinal cognitive decline using the following model:
decline(% per year)=β0+β1×CBVa+β2×Aβ+β3×CBVa×Aβ+β4×Sex+β5×Age+β6×Education+β7×APOE-e4 [1]
RESULTS:
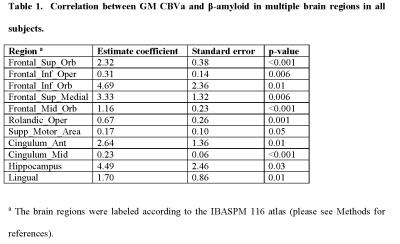
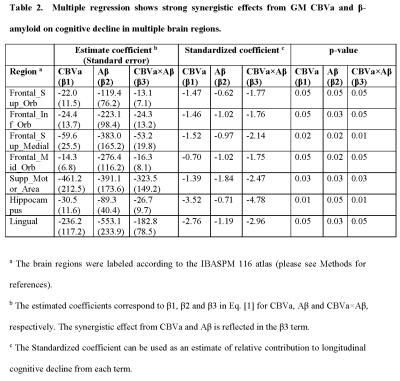
Group comparisons of CBVa values have been reported previously4. Here, the correlation analysis showed that many regions with increased CBVa in subjects with MCI co-localized with regions with increased cortical PiB-ratios. Significant positive correlations between CBVa and PiB-ratios were found in several areas in all subjects (including both MCI and CN subjects, Table 1). In most of these regions, strong synergistic effects from CBVa and PiB-ratio on longitudinal cognitive decline was found using multiple regression (Table 2).DISCUSSION & CONCLUSION:
We report significant spatial correlations between microvascular abnormalities indicated by CBVa and Aβ accumulation measured by Aβ-plaque-load using PiB-PET in various brain regions in subjects with MCI and elderly CN individuals. Multiple regression results suggest that CBVa and Aβ accumulation in several brain regions may have a synergistic effect on longitudinal cognitive decline in elderly subjects at-risk for AD. These regions include several frontal and motor areas, hippocampus, and some other areas that have been implicated as signature regions for neurodegenerative brain change in AD. This implies that for secondary prevention trials in CN individuals with high risk of developing AD, both variables may need to be considered when evaluating outcomes. Further investigation is warranted to validate the findings in a large cohort, and to investigate the underlying mechanism for such interaction between microvascular abnormalities and Aβ accumulation in the brain.Acknowledgements
Funding through KFSP Molecular Imaging Network Zürich (MINZ) and Swiss National Science Foundation, NCRR NIBIB P41 RR15241.References
1. Sperling, R.A., et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & dementia : the journal of the Alzheimer's Association 7, 280-292 (2011).
2. Zlokovic, B.V. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci 12, 723-738 (2011).
3. Balbi, M., et al. Dysfunction of mouse cerebral arteries during early aging. J Cereb Blood Flow Metab 35, 1445-1453 (2015).
4. Hua, J., et al. Abnormal Grey Matter Arteriolar Cerebral Blood Volume and its Association with the Presence of E4 Allele of the Apolipoprotein E (APOE) Gene in Elderly Subjects at Risk for Alzheimer’s Disease (AD) in Proc. 24th Annual Meeting ISMRM (Singapore, 2016).
5. Albert, M.S., et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & dementia : the journal of the Alzheimer's Association 7, 270-279 (2011).
6. Klunk, W.E., et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol 55, 306-319 (2004).
7. van Bergen, J.M., et al. Colocalization of cerebral iron with Amyloid beta in Mild Cognitive Impairment. Scientific reports 6, 35514 (2016).
8. Hua, J., Qin, Q., Pekar, J.J. & Zijl, P.C. Measurement of absolute arterial cerebral blood volume in human brain without using a contrast agent. NMR Biomed 24, 1313-1325 (2011).