2360
Altered Hippocampal Functional Connectivity with PCC in SCI, MCI and AD1Department of Diagnostic Radiology, The University of Hong Kong, Hong Kong, Hong Kong, 2Department of Medicine, The University of Hong Kong, Hong Kong, Hong Kong, 3State Key Laboratory of Brain and Cognitive Sciences, The University of Hong Kong, 4MR Clinical Science, Philips Healthcare, Hong Kong, Hong Kong, 5State Key Laboratory of Brain and Cognitive Sciences, The University of Hong Kong, Hong Kong, Hong Kong, 6Alzheimer's Disease Research Network, The University of Hong Kong, Hong Kong, Hong Kong
Synopsis
Brain imaging research has elucidated the structural and functional changes at the clinical stages of AD and MCI, however, the characteristics of resting-state functional connectivity of SCI are largely unstudied. Hippocampus is among the first regions targeted by AD pathology. In this study, we employed resting state fMRI to study the connectivity between hippocampus and posterior cingulate cortex in SCI, MCI and AD groups. In the preliminary results, the connectivity between hippocampus and PCC in SCI was found stronger than control and other two neurodegenerative groups which may suggest that compensatory mechanisms providing preserved hippocampal pathway in SCI.
Purpose
Much research has investigated the brain activity alterations in patients who are diagnosed with Alzheimer’s disease(AD) and mild cognitive impairment(MCI). However, there are few studies investigating the resting state in subjective cognitive impairment(SCI), which is an early stage that describes the occurrence when a person complains of the impairment of cognitive function. Some research has found that the connections between brain alterations and SCI being established as self-appraising of cognitive functions are more easily influenced compared with objective functions.1 Brain imaging research has elucidated the structural and functional changes at the clinical stages of AD and MCI, however, the characteristics of resting-state functional connectivity of SCI are largely unstudied.2 Intrinsic functional connectivity measured in resting state includes the long-term changes in synaptic efficiency, anatomic connectivity, temporary sources such as synaptic changes due to state of arousal and recent experience,3 and other confounding sources(motion, cardiac and respiratory activity).4 Neurodegenerative disease can easily alter the functional connectivity of specific regions. Hippocampus is among the first regions targeted by AD pathology. 5 In this study, we employed resting state fMRI to study the connectivity between hippocampus and posterior cingulate cortex in SCI, MCI and AD groups.Materials and Methods
Subjects and Image Acquisition:
Eighty-four subjects were recruited initially but five subjects were excluded with head motion. The demographic information was shown in Table 1. The grouping criteria was based on DSM-V and MoCA. All experiments were performed with a Phlips-3T MR scanner using a standard head coil. Structural images were acquired with 3D fast field echo sequence (3D-T1-FFE sagittal). Functional images were collected by using a gradient-echo echo-planar sequence sensitive to blood-oxygen-level-dependent(BOLD) contrast. For this resting-state fMRI, subjects were instructed to open their eyes and view the central fixation cross without thinking of anything in particular.
Data analysis:
The analyses were conducted using the Data Processing Assistant for Resting-State fMRI(DPARSF), which is based on Statistical Parametric Mapping (SPM12) and Resting-state fMRI Data Analysis (REST 1.8). Left hippocampal formation was selected as the seed region,6 and the correlations of time course between hippocampus and other regions were calculated. Two sample T-test was used to compare among four groups (Control, SCI, MCI and AD) for between group comparisons. The threshold was corrected with Alphasim (Two sample t test: p<0.05 and a minimum extent=54 voxels=1458mm3).
Results
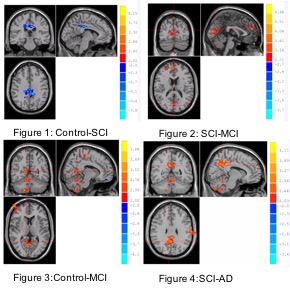
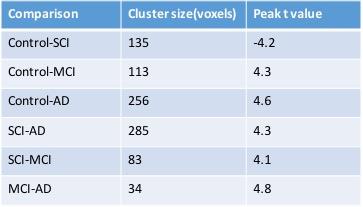
Between group comparisons for different cohorts are shown in the Fig 1-4. The connectivity between PCC and hippocampus is stronger in control compare with MCI and AD. Specifically, the connectivity between hippocampus and PCC is strongest in SCI as compared to other three groups (Table 2 and Fig1-4).Conclusion
The connectivity between hippocampus and PCC in SCI was found stronger than control and other two neurodegenerative groups. The preliminary results may suggest that compensatory mechanisms providing preserved hippocampal pathway in SCI. As the connectivity between the hippocampus and PCC known to be involved in episodic memory indicates the functions consistent with a trait-level neurobiological measure which predicts associative memory ability,4 the connection between hippocampus and PCC may provide insights into the pathophysiological changes in SCI, MCI and AD. More longitudinal studies are needed to validate these results, especially the conversion of SCI to MCI on clinical follow-up.Acknowledgements
No acknowledgement found.References
1. Stewart R. Subjective cognitive impairment. Curr Opin Psychiatry. 2012;25:445-450
2. Erk S, Spottke A, Meisen A, Wagner M, Walter H, Jessen F. Evidence of neuronal compensation during episodic memory in subjective memory impairment. Arch Gen Psychiatry. 2011;68:845-852
3. Vaisvaser S, Lin T, Admon R, Podlipsky I, Greenman Y, Stern N, et al. Neural traces of stress: Cortisol related sustained enhancement of amygdala-hippocampal functional connectivity. Front Hum Neurosci. 2013;7:313
4. Touroutoglou A, Andreano JM, Barrett LF, Dickerson BC. Brain network connectivity-behavioral relationships exhibit trait-like properties: Evidence from hippocampal connectivity and memory. Hippocampus. 2015;25:1591-1598
5. Braak H, Braak E. Staging of alzheimer's disease-related neurofibrillary changes. Neurobiology of Aging. 1995;16:271-278
6. Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain's default network. Neuron. 2010;65:550-562
Figures