2349
Using Calibrated Proton Density Imaging to Measure Blood-Brain Partition Coefficient in Aging and Alzheimer's Disease Mice1Biomedical Engineering, University of Kentucky, Lexington, KY, United States, 2Biomedical Engineering, University of Kentucky, KY, United States, 3Magnetic Resonance Imaging and Spectroscopy Center, 4Sanders-Brown Center on Aging, University of Kentucky, KY, United States, 5Molecular and Biomedical Pharmacology, University of Kentucky, 6Pharmacology and Nutritional Sciences, University of Kentucky
Synopsis
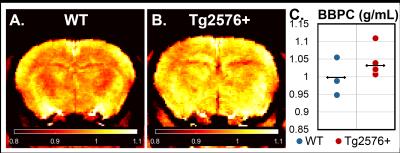
In this study we determine the blood-brain partition coefficient (BBPC) in aging C57Bl6/N mice and the transgenic 129S6/Tg2576 mouse model of Alzheimer’s disease using a calibrated proton density imaging approach. Aging mice demonstrate a 5.5% reduction in BBPC compared to young mice (0.94±0.04 mL/g vs 0.99±0.04 mL/g, p = 0.02), however Tg2576+ mice preliminarily demonstrate an elevated BBPC compared to wild-type controls (01.03±0.04 mL/g vs 1.00±0.05 mL/g). These high quality BBPC maps acquired much faster than previously reported could potentially be used to correct cerebral blood flow measurements derived from arterial spin labeling.
Target Audience
Individuals interested in quantitative arterial spin labeling in mouse models of aging.Purpose
In the present study, we determine the blood-brain partition coefficient (BBPC) in aging C57Bl6/N mice and the transgenic 129S6/Tg2576 mouse model of Alzheimer’s disease using a calibrated proton density imaging approach.1 This parameter is an important coefficient in the quantification of cerebral blood flow (CBF) derived from arterial spin labeling (ASL) acquisitions. Previous studies have shown both regional and age-related differences in BBPC in humans, yet the current consensus in the field does not correct for these differences but instead assumes a single constant value for all regions and all patients.2 Arterial spin labeling has become particularly relevant in the study of brain aging where it has been used to image the vascular dysfunction that occurs with advanced age. In Alzheimer’s disease it has also shown sensitivity to the vascular dysfunction which precedes amyloid and tau pathologies. This has been recapitulated in small animal models such as the 129S6/Tg2576 mice which have the human Swedish amyloid precursor protein (hAPP) mutation3. However, the limitations of small animal scanners and the inherent low signal of ASL techniques require quantification models to be as precise as possible. Furthermore, any uncorrected variation in BBPC could potentially bias CBF measurements. For this reason, we test the hypothesis that BBPC will be reduced in aged C57Bl6/N mice and transgenic 129S6/Tg2576 mice.Methods
Imaging Protocol- Male C57Bl6/N wild type mice aged 3 months (n=8) and 12 months (n=8) as well as male 12-month-old 129S6/Tg2576 (n=6) with their 129S6 wild type controls (n=3) were imaged using a 7T Bruker ClinScan (Bruker Biospin, Ettlingen, Germany) with a 39mm diameter birdcage transmit/receive coil. Inside the coil was placed a series of phantoms with 0, 10, 20, 30, and 40% deuterium oxide in water that were also doped with gadobutrol (Gadavist, Bayer Healthcare Pharmaceuticals, Whippany NJ, USA, 0.07 mM) such that the T1 was approximately 2.0s. Blood was drawn from the facial vein of each subject and placed in a capillary tube alongside the deuterated phantoms. A series of image stacks were acquired with a phase-spoiled, FLASH-GRE sequence (FOV = 2.8cm x 2.8cm, matrix = 256 x 256, slice thickness = 1mm, number of slices = 10, flip angle = 90°) with a very short TE (3.2ms) and 6 different TR values (125, 187, 250, 500, 1000, 2000ms).1
Image Analysis- For each transverse slice, the TR-series was fit to the mono-exponential recovery curve S = M0*[1-e^(TR/T1)] in a voxel-wise manner yielding maps of both apparent T1 and relative proton density, M0. The relative M0 maps were calibrated to a regression line of the average M0 values in regions of interest (ROIs) drawn in the deuterated phantoms. Therefore, the calibrated M0 values represent the percent water content of each voxel. The BBPC value is then calculated by dividing by the average M0 value in the blood sample and the average density of brain tissue, i.e. BBPC = M0,brain/(M0,blood * 1.04g/mL). An ROI was then drawn manually for each transverse slice excluding any susceptibility artifacts. BBPC values were averaged over all ROIs for each mouse.
Results
The calibrated proton density imaging protocol was able to produce high resolution, low noise maps of BBPC (Figs. 1 & 2) despite reducing scan time from 2 hours, as in Leithner et al., to 25 minutes. The 12 month old mice demonstrated a 5.5% reduction in BBPC (µ= 0.94±0.04 mL/g) compared to the 3 month old mice (µ= 0.99±0.04 mL/g, p = 0.02) (Fig. 1-C). Preliminarily, the Tg2576+ mice demonstrate an elevated BBPC (µ= 01.03±0.04 mL/g) compared to WT (µ= 1.00±0.05 mL/g) (Fig. 2-C), though more subjects are needed.Discussion/Conclusion
The variability of BBPC values from these data demonstrates the potential error in assuming a constant value for all patients when calculating CBF. When measuring CBF in aging mice, failing to correct for the reduced BBPC will overestimate CBF resulting in reduced sensitivity. However, it appears that in the 129S6/Tg2576 model the elevated BBPC may be a confounding factor. Scan time can be reduced further by reducing the resolution to match ASL acquisitions making this technique a potentially viable method of correcting CBF measures for differences in BBPC.Acknowledgements
- ST supported by the F. Joseph Halcomb III MD Fellowship for Engineering in Medicine
- Funding from NIH/NIA Grant # K01AG040164 to AL
References
1. Leithner C, Muller S, Fuchtemeier M, Lindauer U, Dirnagl U and Royl G. Determination of the brain-blood partition coefficient for water in mice using MRI. J Cereb Blood Flow Metab. 2010;30:1821-4.
2. Alsop DC, Detre JA, Golay X, Gunther M, Hendrikse J, Hernandez-Garcia L, Lu H, MacIntosh BJ, Parkes LM, Smits M, van Osch MJ, Wang DJ, Wong EC and Zaharchuk G. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med. 2015;73:102-16.
3. Rustay NR, Cronin EA, Curzon P, Markosyan S, Bitner RS, Ellis TA, Waring JF, Decker MW, Rueter LE and Browman KE. Mice expressing the Swedish APP mutation on a 129 genetic background demonstrate consistent behavioral deficits and pathological markers of Alzheimer's disease. Brain Res. 2010;1311:136-47.
Figures