2333
Could ASL separate MCS from VS in patients with DOC?1Radiology Dept., PLA Army General Hospital, Beijing, People's Republic of China, 2Neurosurgery Dept., PLA Army General Hospital, Beijing, People's Republic of China, 3Academy of Military Medical Sciences, Beijing, People's Republic of China
Synopsis
This study used 3D pseudo-continuous arterial spin labeling (pcASL) to compare cerebral blood flow (CBF) patterns in minimally conscious state (MCS) patients with those in vegetative state (VS) ones. The results identified different CBF patterns within specific brain regions in VS patients compared with MCS. ASL may serve as an adjunctive method to separate MCS from VS in DOC patients, and could be used in longitudinal assessments of patients with severe brain injuries.
PURPOSE:
Coma, vegetative state (VS), and minimally conscious state (MCS) were collectively termed disorders of consciousness (DOC). A subset of coma patients develops a prolonged impairment in consciousness, such as the VS or MCS. Diagnostic error is common among patients with VS and MCS.1 Because of variable behavior observed at the bedside, approximately 30–40% of people diagnosed with VS actually retain conscious awareness.2 The purpose of this paper is to use 3D pseudo-continuous arterial spin labeling (pcASL) to compare cerebral blood flow (CBF) patterns in MCS patients with those in VS in an observational study design.METHODS:
Subjects meeting MCS criteria VS were identified. Two post labeling delay time (PLD) pcASL sequences on 3.0-Tesla MR scanner were performed with both groups of patients in the resting awake state. After registration to T1WI structure imaging, multiple brain regions of interest of ASL CBF map were automatically separated based on Automated Anatomical Labeling (AAL) brain template. Then the average CBF value of every brain region was calculated and compared between MCS and VS groups with t-tests.RESULTS:
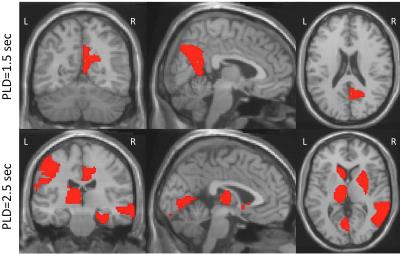
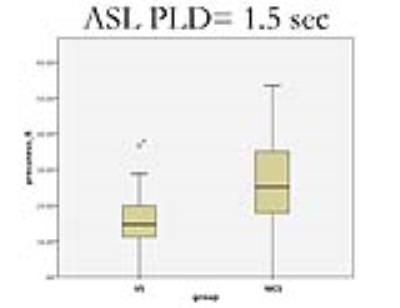
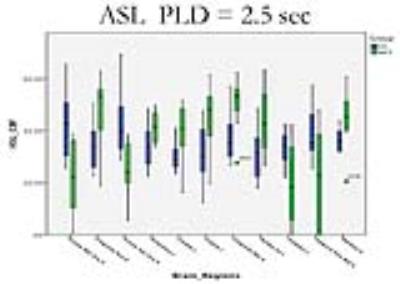
Sixteen patients with VS were identified, with ages ranging from 25 to 69 years. Nine patients met the MCS criteria ranged in age from 23 to 61 years. Etiologies included traumatic brain injury, stroke, or hypoxic-ischemic encephalopathy. All patients received two ASL studies. Compared with VS patients, the regional CBF with PLD 1.5 sec for MCS had a pattern of relatively increased CBF in the region of precuneus right (P<0.05). The regional CBF with PLD 2.5 sec for MCS had a pattern of relatively increased CBF in the regions including R Cingulum_Post, L Postcentral, L Caudate, L Lingual, R Cingulum_Mid, L Parietal_Inf, and R Pallidum (P<0.05), and relatively decreased CBF in the regions including R Frontal_Mid_Orb, R Frontal_Sup_Orb, L Thalamus, and R Temporal_Pole_Mid (P<0.05).(Shown on Fig.1, 2, and 3).DISCUSSION:
The regionally decreased CBF among VS subjects compared with MCS in our study is consistent with earlier observations of reduced activity in midline structures measured with resting state fMRI or PET.3 Human and animal studies indicate a strong linkage between CBF and glucose metabolic rate.4,5 The precuneus is the posterior region of the medial parietal cortex and represents one of the most highly connected and metabolically active regions of the brain. It is believed to be involved in self-referential tasks. In several previous studies, when compared with subjects in the MCS, patients in VS had proportionally greater reductions in activity in the precuneus and in the posterior cingulate cortex (PCC).3,6 Moreover, the reduced connectivity affected mostly the precuneus, a main node of the default mode network (DMN) considered to be a critical hub for consciousness.7CONCLUSION:
We identified a selective reduction or increase of CBF within specific brain regions in VS patients compared with MCS. ASL may serve as an adjunctive method to separate MCS from VS in DOC patients, and assess functional reserve in patients recovering from severe brain injuries. And because of its advantages of speed and ease of acquisition and its ability to provide precise quantitative CBF, ASL could be used in longitudinal assessments of patients with severe brain injuries.Acknowledgements
No acknowledgement found.References
1. Giacino JT, Fins JJ, Laureys S, Schiff ND. Disorders of consciousness after acquired brain injury: the state of the science. Nat Rev Neurol. 2014 Feb;10(2):99-114.
2. Liu AA, Voss HU, Dyke JP, Heier LA, Schiff ND. Arterial spin labeling and altered cerebral blood flow patterns in the minimally conscious state. Neurology. 2011 Oct 18;77(16):1518-23.
3. Hannawi Y, Lindquist MA, Caffo BS, Sair HI, Stevens RD. Resting brain activity in disorders of consciousness: a systematic review and meta-analysis. Neurology. 2015 Mar 24;84(12):1272-80.
4. Smith AJ, Blumenfeld H, Behar KL, Rothman DL, Shul- man RG, Hyder F. Cerebral energetics and spiking fre- quency: the neurophysiological basis of fMRI. Proc Natl Acad Sci USA 2002;99:10765–10770.
5. Ma Y, Eidelberg D. Functional imaging of cerebral blood flow and glucose metabolism in Parkinson’s disease and Hun- tington’s disease. Mol Imaging Biol 2007;9:223–233.
6.Kim YW, Kim HS, An YS. Brain metabolism in patients with vegetative state after post-resuscitated hypoxic-ischemic brain injury: statistical parametric mapping analysis of F-18 fluorodeoxyglucose positron emission tomography. Chin Med J (Engl) 2013;126:888–894.
7.Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005 May 29;360(1457):1001-13.
Figures