2326
Dynamic Quantitative Imaging of Sodium During Migraine Onset and Propagation at 21.1 T1Center for Interdiscplinary Magnetic Resonance, National High Magnetic Field Laboratory, Tallahassee, FL, United States, 2Chemical & Biomedical Engineering, Florida State University, Tallahassee, FL, United States, 3Molecular Neurology Program, Huntington Medical Research Institutes, Pasadena, CA, United States
Synopsis
The brain allocates >50% of its energy reserves to the regulation of sodium homeostasis, indicating the critical importance of sodium and its fluxes in normal brain and neurological disorders. The purpose of this study was to evaluate in vivo 23Na in the brain using a rat model of migraine induced by nitroglycerin injection. The ultra-high field of 21.1 T was employed to quantify alterations in bulk sodium during and following the onset of central sensitization related to migraine. Multi-slice 2D CSI of sodium were acquired longitudinally to identify localized increases in sodium concentrations during a 3-h period following sensitization induction.
Purpose
Ionic instability and dysfunctional metabolic regulation is implicit in migraineurs. The brain allocates over 50% of its energy reserves on a cellular basis to the regulation of sodium homeostasis; therefore, the alteration of sodium concentrations and fluxes related to these distributions are critical1. Accordingly, it is hypothesized that migraineurs have a distinct and prevailing mechanism that induces excess sodium to be pumped into the extracellular space, resulting in neuronal excitability that is sustained with the onset and progression of migraine. More specifically, the purpose of this study was to evaluate dynamic in vivo 23Na fluxes in the brain using a migraine analogue in rodents2. The ultra-high field of 21.1 T was utilized to allow for higher spatial and temporal resolution to quantify bulk sodium distributions during and following the onset of central sensitization (CS) to develop a novel time course of > 3 h. This longitudinal time course allowed for investigation of bulk sodium changes during the onset and propagation of CS, while rigorously accounting for any cyclical or circadian variations in biological sodium.Materials and Methods
Animal Model: A total of 19 male Sprague-Dawley rats were used in this study. While in the MRI scanner and sedated, they were administered in situ with an IP injection of either 10 mg/kg of NTG (n=6) to induce a migraine analogue or saline (n=6), vehicle (n=3) or naïve (n=4) as controls and baselines.
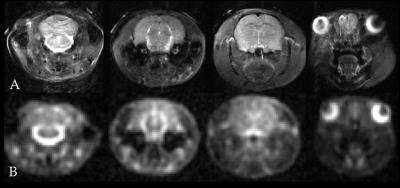
MRS protocol: All scans were performed using the 21.1-T, 900-MHz ultra-widebore at the National High Magnetic Field Laboratory, Tallahassee, FL. A home built double tuned 23Na/1H radio frequency (RF) linear birdcage coil was used to acquire in vivo 23Na and 1H data. For 1H references and 23Na imaging, four simultaneously acquired 3-mm thick coronal slices were located 8.5 mm anterior, 1 mm posterior, 7 mm posterior and 11 mm posterior to Bregma to generate image datasets that covered the eyes and olfactory bulb (slice 1); the third ventricle, neocortex and muscle (slice 2); the fourth ventricle (slice 3); and the brainstem, cerebellum and cisterna magna (slice 4), respectively. Slice-selective sodium images were acquired using a multi-slice FID-based Chemical Shift Imaging (CSI) sequence yielding an in-plane acquisition resolution of 1.1 x 1.1 mm and through-plane resolution of 3-mm. A weighted Gaussian sampling profile with 500 phase encodes was used. This excitation yielded maximum signal for a TR=180 ms, and with 6 averages, the complete dataset was acquired in 9 min. A total of twenty-seven repeated scans were acquired starting from pre-injection (at 11:00 am on each experiment day), with 1-h of baseline scanning, followed by 3-h post injection scanning (initiated at 12:00 pm).
Data Analysis: The 2D CSI datasets were zero-filled to 512 points in the spectral dimension with 20-Hz exponential line broadening was applied prior to transformation. Data were also sampled to a 64x64 matrix in the spatial dimensions to achieve an image resolution of 0.47x0.47x3.0 mm (Figure 1). Images were manually segmented with ROIs placed in the eyes, olfactory bulb, third ventricle, neocortex, fourth ventricle, cerebellum, brainstem and cisterna magna along with a muscular region in the rat jaw to serve as an internal control for 23Na signal. Mean signal intensities in the ROIs were calibrated to phantom measurements to estimate total tissue sodium concentration and are presented as a percent change from baseline concentrations.
Results
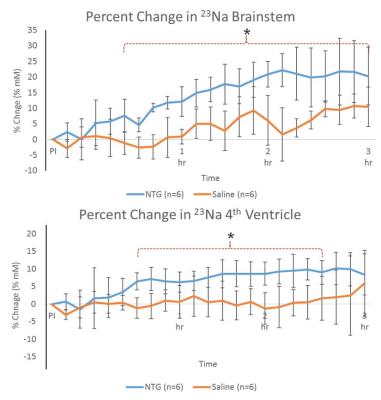
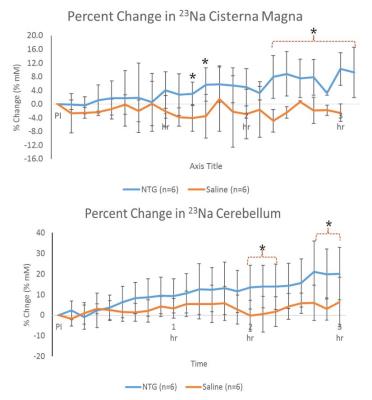
Shown in Figure 2, increased 23Na signals were evident for NTG-treated animals in the brainstem and 4th ventricle immediately after injection, gaining significance about 30-45 min post-NTG injection. The cisterna magna, cerebellum and 3rd ventricle (Figure 3) showed increasing trends of 23Na concentration post-injection, gaining delayed significance at >1.5 h. Naive and vehicle cohorts were similar to saline, and showed no significant change over the time course..
These dynamic changes (rapid increases in sodium concentration compared to the controls) indicate that the mechanism of CS is dependent on, specific to, and localized in certain anatomical regions. Increasing in 23Na immediately after injection, the brainstem and 4th ventricle appear to be the first to gain significance followed by cisterna magna, indicating the likelihood of a cascade of events leading to the integration of nociceptive stimulation. In contrast, delayed cerebral impacts could be reflective of vestibular impacts of CS.
Conclusions
Uniquely, brain 23Na fluctuations have not been evaluated previously with the present spatial and temporal resolution spanning both acute and delayed onset of central sensitization. Preliminary analysis of data provides evidence for the onset and progression of CS as a brain-based disturbance. These time dependent fluctuations could provide prominent insight into dysregulations of migraine and subsequent therapeutic measures.Acknowledgements
This work was supported by the NIH (R01-NS072497 to MGH) and User Collaborations Grant Program (to SCG) from the National High Magnetic Field Laboratory, which is funded by the NSF (DMR-1157490) and the State of Florida.References
1. Harrington M, Fonteh A, Cowan R, Perrine K, Pogoda J, Biringer R, Huhmer A. Cerebrospinal fluid sodium increases in migraine. Headache 2006;46(7):1128-35.
2. Harrington M, Chekmenev E, Schepkin V, Fonteh A, Arakaki X. Sodium MRI in a rat migraine model and a NEURON simulation study support a role for sodium in migraine. Cephalalgia 2011;31(12):1254-65.
Figures