2320
Mapping brain functional alterations in chemotherapy-treated breast cancer women using resting-state fMRI1Department of Medical Imaging and Radiological Sciences, Chung Shan Medical University, Taichung, Taiwan, 2School of Medicine, Chang Gung University, Taoyuan, Taiwan, 3Department of Psychiatry, Chang Gung Memorial Hospital, Chiayi, Taiwan, 4Breast Center, Taichung Tzu Chi Hospital, Taichung, Taiwan, 5Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan, 6Department of Medical Imaging, Chung Shan Medical University Hospital, Taichung, Taiwan
Synopsis
Breast cancer (BC) is one of the common public health problems, and chemotherapy was the major treatment for breast cancer. The previous study showed abnormal brain function was associated with the late effects of chemotherapy (5 to 10 years). The purpose of our study was to evaluate the early effects of post-chemotherapy BC patients (in 6 months). We investigated the resting-state functional differences between post-chemotherapy BC patients and healthy control. Our results provided the evidence of brain functional changes in women with breast cancer and highlight the importance of the breast cancer-related chemotherapy.
Introduction
Breast cancer is one of the common public health problems, and chemotherapy was the major treatment for breast cancer. The previous study showed abnormal brain function was associated with chemotherapy treatment 1, and mainly discussed the late effects of chemotherapy 2. They showed breast cancer patient with chemotherapy had significantly cognitive function decline with regard to verbal ability and visuospatial ability 3, or worse function in the prefrontal cortex or premotor cortex 4. Our study aimed to evaluate the early effects of post-chemotherapy BC patients. We investigated the functional differences between post-chemotherapy BC patients and health controls with mean fractional amplitude of low frequency fluctuations (mfALFF) and mean regional homogeneity (mReHo) by using resting-state fMRI (rs-fMRI) and voxel-based analysis.Methods
Our study included 19 breast cancer patients (post-chemotherapy in 6 months) and 20 healthy controls. All participants were scanned using 1.5T MRI (Area, Siemens, Germany) imaging system with echo planar image (EPI) sequence to obtain resting-state functional images. The fMRI parameters were as follows: TR/TE = 2000/30 ms; voxel size=3.4 X 3.4 X 4 mm3; number of scan=180 and 33 axial slices. The resting-state fMRI raw data were processed with Statistical Parametric Mapping (SPM8, Wellcome Department of Cognitive Neurology, London, UK) and Resting-State fMRI Data Analysis Toolkit (REST1.8, Lab of Cognitive Neuroscience and Learning, Beijing Normal University, China). In preprocessing, the images were corrected for the differences in slice acquisition times and head motion, and the corrected images were spatial normalized to Montreal Neurological Institute (MNI) template. The normalized images were smoothed with a Gaussian kernel of the 6 mm full-width at half maximum (FWHM=6mm). The linear trend of the functional data was then removed, and a band filter with 0.01-0.12Hz was applied. After removing physiological noises, mean fractional amplitude of low frequency fluctuation (mfALFF) and mean regional homogeneity (mReHo) were calculated. Then voxel-based two-sample T-test analysis was used to analyzed mfALFF and mReHo, and age was used as the covariate in the analysis.Results
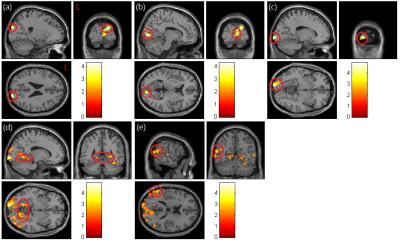
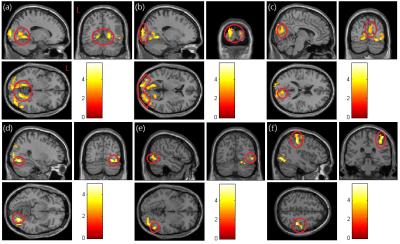
In mfALFF analysis (Fig. 1), our results showed that post-chemotherapy patients have significant lower activation in right superior occipital gyrus (p<0.01) (Fig. 1a), bilateral calcarine (p<0.01) (Fig. 1b, c), bilateral lingual (p<0.03) (Fig. 1d), right middle temporal gyrus (p<0.05) (Fig. 1e) compared with healthy controls. In mReHo analysis (Fig. 2), post-chemotherapy patients have significant lower regional homogeneity in bilateral lingual (p<0.005) (Fig. 2a), bilateral superior, middle and inferior occipital gyrus (p<0.005) (Fig. 2b), right cuneus (p<0.005) (Fig. 2c), right fusiform (p<0.005) (Fig. 2d), middle and inferior temporal gyrus (p<0.005) (Fig. 2e), and right postcentral (p<0.005) (Fig. 2f) compared with healthy controls.Discussion
The bilateral lingual showed lower mfALFF and mReHo in post-chemotherapy BC patients compared with healthy control. The lingual gyrus is a structure in the visual cortex that plays an important role in the vision and associates memorization and activation 5. Visual memory dysfunction has been shown in cases where the lingual gyrus has been damaged 6. Previous studies have shown the effect of neurocognitive by chemotherapy including visual and verbal memory, concentration and motor coordination 3. Other regions we found, like cuneus and calcarine sulcus, have significant lower mfALFF and mReHo in BC patients, which are parts of the visual cortex. The cuneus is involved in processing visual information and is part of the dorsal and ventral visual streams. Some evidence showed that visual system injury caused by chemotherapy may be the main reason for abnormal neurocognitive 7.Conclusion
Our results of mfALFF and mReHo showed lower activation and regional homogeneity of several brain regions in the post-chemotherapy breast cancer patients compared to healthy controls. Our results provided the evidence of brain functional changes in women with breast cancer and highlight the importance of the breast cancer-related chemotherapy.Acknowledgements
This study was supported in part by the research programs NSC103-2420-H-040-002 and MOST104-2314-B-040-001, which were sponsored by the Ministry of Science and Technology, Taipei, Taiwan.References
1. Kesler SR, Bennett FC, Mahaffey ML, et al. Regional brain activation during verbal declarative memory in metastatic breast cancer. Clin Cancer Res. 2009; 15(21): 6665-6673.
2. Silverman DH, Dy CJ, Castellon SA, et al. Altered frontocortical, cerebellar, and basal ganglia activity in adjuvant-treated breast cancer survivors 5-10 years after chemotherapy. 2007; 103(3): 303-311
3. Jim HS, Phillips KM, Chait S, et al. Meta-analysis of cognitive functioning in breast cancer survivors previously treated with standard-dose chemotherapy. J Clin Oncol. 2012; 30(29): 3578-3587.
4. Kesler SR, Kent JS, O’Hara R, et al. Prefrontal cortex and executive function impairments in primary breast cancer. Arch Neurol. 2011; 68(11): 1447-1453.
5. Leshikar ED, Duarte A, Hertzog C. Task-selective memory effects for successfully implemented encoding strategies. PLoS ONE. 2012; 7(5): e38160.
6. Bogousslavsky J, Miklossy J, Deruaz JP, et al. Lingual and fusiform gyri in visual processing: a clinico-pathologic study of superior altitudinal hemianopia. J Neurol Neurosurg Psychiatry. 1987; 50(5): 607-614.
7. Al-Tweigeri T, Nabholtz JM, Mackey JR. Ocular toxicity and cancer chemotherapy. A review. Cancer. 1996; 78(7): 1359-1373.
Figures