2318
No Evidence of Increased Iron Accumulation in the Deep Gray Matter of Patients with Amyotrophic Lateral Sclerosis (ALS)1Department of Neurology, Medical University of Graz, Graz, Austria, 2Division of Neuroradiology, Vascular and Interventional Radiology, Department of Radiology, Medical University of Graz, Graz, Austria
Synopsis
Iron accumulation in deep gray matter occurs during normal aging, but has also been observed in several neurodegenerative diseases. To investigate if this still holds true for ALS, we aimed at assessing the iron content in deep gray matter structures of 24 ALS patients with quantitative susceptibility mapping and its relation to 28 controls. We did not find any significant differences in iron levels between ALS patients and controls. This may indicate that iron accumulation in deep gray matter is a specific feature that is not associated with neurodegenerative diseases in general, but rather reflects more specific degenerative processes.
Introduction
Amyotrophic lateral sclerosis (ALS) presents an invariably fatal, progressive neurological disorder and is characterized by degeneration of the lower and upper motor neuron system. While iron accumulates in deep gray matter (DGM) during normal aging,1 increased iron concentration has been suggested as an epiphenomenon of several neurodegenerative diseases, such as Alzheimer, Parkinson's, and Huntington disease.2 If this also holds true for ALS is still unclear. Therefore, in this study we aimed at assessing the iron content in DGM structures of ALS patients with quantitative susceptibility mapping (QSM) and to comparing the results to age-matched controls.Methods
24 ALS patients (age range 34-78 years, mean ALS functional rating scale score (ALSFRS) = 38.25) and 28 age-matched healthy controls (HC) underwent a comprehensive clinical examination and MRI at 3 Tesla (Tim Trio, Siemens Medical Systems). Structural scans were acquired with an MPRAGE sequence with 1 mm isotropic resolution and whole brain coverage (TR/TE/TI/FA = 1.9 s/2.19 ms/900 ms/9°). A 3D spoiled gradient echo sequence (TR/TEfirst/FA = 35 ms/4.92 ms/15°) with 6 equally spaced echoes (bipolar readout gradient, inter-echo spacing = 4.92 ms, resolution = 0.9x0.9x2 mm³, FOV = 230 mm) was used to quantitatively map the magnetic susceptibility. QSM maps were generated with a recently proposed algorithm3 based on dipole inversion with total generalized variation. All QSM maps were normalized by using the cerebrospinal fluid as reference. DGM structures (putamen, globus pallidus, caudate nucleus, and thalamus) were fully automatically segmented using FSL FIRST4 and the high resolution T1-weighted scan. All regions were then linearly transformed onto the QSM-maps and binarized using a 90% threshold. A student t-test was used to assess the differences between ALS patients and HCs. Univariate linear regression analysis served to investigate the relationship of iron levels with disease duration and severity as indicated by the ALSFRS.Results
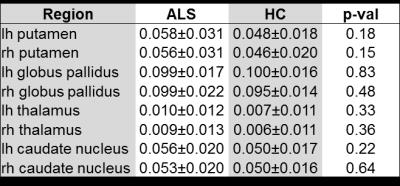
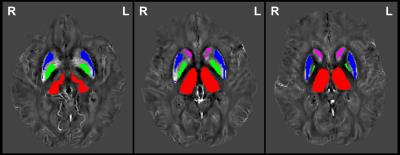
Figure 1 illustrates the analyzed DGM regions as overlays on the QSM maps of an ALS patient. The respective susceptibility values (mean χ ± SD χ) of ALS patients and HCs are provided in Table 1. As can be seen in the aforementioned there are no significant differences in susceptibilities as a reflection of iron concentration between both groups. We also found no significant correlation of iron levels with disease duration and ALS severity.Discussion and Conclusion
Applying a sensitive and reliable technique for iron mapping, we did not find abnormal iron levels in the DGM nuclei of ALS patients. This is in contrast to an earlier ALS study,5 which suggested an increase of iron in the caudate nucleus when performing effective transversal relaxation rate analysis. However, our results are in line with other studies which also observed no differences for DGM structures between ALS patients and controls on T2*-weighted and susceptibility weighted images.6,7 Furthermore, the absence of a correlation between iron level and clinical measures of disease severity and duration is supported by a recent study.7 This indicates that iron accumulation in DGM structures is not a general observation in neurodegenerative disorders, but rather specific for certain diseases.Acknowledgements
No acknowledgement found.References
1. Hallgren B, Sourander P. The Effect of Age on the Non-Haemin Iron in the Human Brain. J Neurochem 1958;3:41–51
2. Berg D, Youdim MBH. Role of Iron in Neurodegenerative Disorders. Top Magn Reson Imaging 2006;17:5–17.
3. Langkammer C, Bredies K, Poser BA, et al. Fast quantitative susceptibility mapping using 3D EPI and total generalized variation. Neuroimage 2015;111:622–30.
4. Patenaude B, Smith SM, Kennedy DN, et al. A Bayesian
model of shape and appearance for subcortical brain segmentation. Neuroimage
2011;56:907–22.
5. Langkammer C, Enzinger C, Quasthoff S, et al. Mapping of iron deposition in conjunction with assessment of nerve fiber tract integrity in amyotrophic lateral sclerosis. J Magn Reson Imaging 2010;31:1339–45.
6. De Reuck JL, Deramecourt V, Auger F, et al. Iron deposits in post-mortem brains of patients with neurodegenerative and cerebrovascular diseases: a semi-quantitative 7.0 T magnetic resonance imaging study. Eur J Neurol 2014;21:1026–31.
7. Yu J, Qi F, Wang N, et al. Increased iron level in motor cortex of amyotrophic lateral sclerosis patients: An in vivo MR study. Amyotroph Lateral Scler Front Degener 2014;15:357–61.
Figures