2317
Network-dependent changes in functional connectivity at 7 tesla in young adults with Down syndrome1Imaging Institute, The Cleveland Clinic, Cleveland, OH, United States, 2Case Western Reserve University, Cleveland, OH, United States, 3University Hospitals, Cleveland, OH, United States
Synopsis
This works uses 7 tesla MRI to assess resting state functional connectivity (rs-fMRI) in young adults with Down syndrome (DS). As compared to matched controls, individuals with DS show increased rs-fMRI in the default mode network, but decreased rs-fMRI in a network related to executive function.
Introduction
Down syndrome (DS) is the most common non-inherited genetic cause of developmental disability, leading to a range of well-described cognitive and physiological characteristics, including an increased incidence of dementia [1]. Neuroanatomical features related to DS have been fairly consistently described, but reports of neurofunctional changes are few. A more complete understanding of neurofunctional changes in DS has the potential to increase both our understanding of the pathophysiology of DS and to provide a “road map” of changes related to typical aging and those that are the consequence of a disease process such as dementia.
Recently, a number of studies have used low-frequency fluctuations in the blood oxygen level dependent (BOLD) timeseries during rest (rs-fMRI) to identify disruptions in intrinsic brain connectivity in DS [2,3,4]. Here we add to this literature with a preliminary analysis of rs-fMRI in young adults with DS at 7 tesla. We focus on the default mode network (DMN), which shows changes related to Alzheimer’s disease and increased amyloid deposition [5,6], and on the fronto-parietal network, related to measures of executive function [7].
Methods
4 young adults with DS (mean age 18.8 ± 2.2, 2 males) and 4 age-matched controls (mean age 19.5 ± 3.1, 3 males) were scanned in an IRB-approved protocol.
MRI acquisition: Scans were acquired on a Siemens 7T Magnetom with SC72 gradient (Siemens Medical Solutions, Erlangen) using a 32-channel head coil (Nova Medical). A whole-brain anatomical MP2RAGE (0.75mm isotropic voxel size) and a rs-fMRI scan were acquired. Rs-fMRI acquisition parameters were as follows: 132 repetitions of 81 1.5mm thick axial slices acquired with TE/TR=21ms/2800ms, matrix 160x160, FOV 210mm x 210mm, receive bandwidth = 1562 Hz/pixel. Subjects were instructed to keep their eyes closed during scans.
Data analysis: Rs-fMRI scans were corrected for motion and physiologic noise, detrended, and lowpass filtered [8,9]. Using a previously published method [10], 9-voxel in-plane seeds were placed in individual datasets to evaluate the strength of connectivity in the default mode network (posterior cingulate cortex) and the fronto-parietal network (left dorsal lateral prefrontal gyrus). Correlation measures were normalized to z-scores using whole-brain correlation maps [11]. For each network, rs-fMRI maps were transformed into common space and averaged for each group. Group maps were added to form a conjunction mask, so that a region that was significant in either group was included in the mask for that network. For each network, a voxel-wise paired t-test of all regions in the mask was used to determine areas showing group differences. A correction for multiple comparisons was calculated using the AFNI AutoCorrelation Function method.
Whole brain white matter, cortical grey matter, and ventricle size was estimated using the MP2RAGE in Freesurfer. Volumes were normalized by dividing by total brain volume.
Results
DS and control subjects did not show significant differences in age (p=0.215). Ventricle volume was larger in individuals with DS (p = 0.021). No other volumetric measure reached significance.
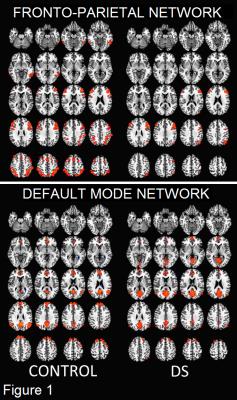
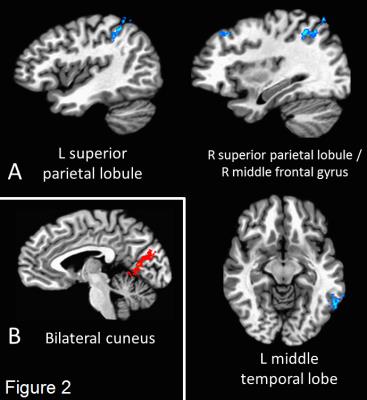
Figure 1 shows the group-averaged rs-fMRI maps for each network (p < 0.01, corrected). Four regions within the fronto-parietal network showed significant group differences (p < 0.05, corrected): bilateral superior parietal lobule, left middle temporal lobe, and the right middle frontal gyrus (BA 8). All regions showing decreased connectivity in the DS group (Figure 2A). Only the bilateral cuneus showed significant group differences in the DMN (Figure 2B), with increased connectivity in the DS group.
Connectivity measures were not significantly related to volumetrics, though the strength of connectivity from the left DLPFC to the right superior parietal lobule was weakly related to cortical grey matter volume (r = 0.946, p = 0.053).
Discussion and Conclusion
In the current study, individuals with DS show increased rs-fMRI strength to the posterior cingulate compared to healthy controls, in agreement with our previous report, and with reports of widespread increases in brain synchrony in those with DS [12,2]. However, in a traditional “task-positive” network we find decreases in connectivity in DS. Future work will increase the sample size of this work and will compare rs-fMRI to cognitive measures.
This work represents the first assessment of rs-fMRI in DS at ultra-high field strength. We find that individuals with DS show increased connectivity in the DMN and decreased connectivity in the frontoparietal network as compared to controls. Additional work is needed to clarify the relationship between imaging measures, cognitive ability, and disease metrics.
Acknowledgements
This work was supported by The Alana Foundation USA.References
[1] Roizen & Patterson, (2003) Down’s Syndrome. Lancet. 361: 1281-1289.
[2] Anderson et al., (2013) Abnormal brain synchrony in Down Syndrome. Neuroimage Clin. 2:703-715.
[3] Vega et al., (2015) Resting-State Functional Connectivity in Individuals with Down Syndrome and Williams Syndrome Compared with Typically Developing Controls. Brain Connectivity. 5(8): 461-475.
[4] Pujol et al., (2015) Anomalous brain functional connectivity contributing to poor adaptive behavior in Down syndrome. Cortex. 64:148-156.
[5] Zhang et al. (2010) Resting brain connectivity: changes during the progress of Alzheimer disease. Radiology. 256:598-606.
[6] Sheline et al. (2010) Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol Psychiatry. 67:584-87.
[7] Niendam et al. (2013) Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions Cogn Affect Behav Neurosci. 12(2): 241–268.
[8] Beall EB and Lowe MJ. (2014) SimPACE: generating simulated motion corrupted BOLD data with synthetic-navigated acquisition for the development and evaluation of SLOMOCO: a new, highly effective slicewise motion correction. Neuroimage. 101:21-34.
[9] Glover et al. (2000) Image-Based Method for Retrospective Correction of Physiological Motion Effects in fMRI: RETROICOR. Magnetic Resonance in Medicine. 44:162-67.
[10] Lowe et al. (2014) Anatomic connectivity assessed using pathway radial diffusivity is related to functional connectivity in monosynaptic pathways. Brain Connectivity. 4(7):558-65.
[11] Lowe et al. (1998) Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. NeuroImage. 7(2):119-32.
[12] Koenig et al. (2015, June) Posterior cingulate connectivity is increased in adults with Down syndrome. Poster presented at the meeting of the Organization for Human Brain Mapping, Honolulu, Hawaii.
Figures