2315
White matter microstructural changes in Rett syndrome: a whole brain tract-specific analysis1National Taiwan University, Taipei, Taiwan, 2National Taiwan University, Taiwan, 3National Taiwan University Hospital, 4National Taiwan University
Synopsis
Rett syndrome (RTT) is characterized by trajectory changes in cognition and motor functions. To understand the disease-specific pathologic changes of each
Purpose
Rett syndrome is a neurodevelopmental disease that primarily affects girls. The prevalence is approximately 1/10,000 to 1/23,000 girls worldwide.1 Clinically, patients are seemingly normal before 6-18 months, and then develop progressive severe problems with communication, cognition and neurodevelopment. However, some of the abilities will improve such as eye gaze and repetitive hand movements after few years.2 Researches in neuropathology presented marked changes in total brain volume. The reductions were not attributed to atrophy but to poor neuronal soma size and dendritic arborizations.3 More recently, neuroimaging studies indicated that a widespread decrease in gray matter, and relative preservation in the occipital region.4 DTI studies showed the reductions in several regions of white matter by ROI analysis.5,6 Most of previous studies focused on children rather than adolescents and younger adults. Thus, the findings of data have been variable and insufficient to explain the development of whole brain microstructures in older patients. Based on previous studies, the purpose of our study is to explore the structure-specific pathologic changes of each individual tract bundle in younger adult of RTT.Methods
Subjects: 12 patients (age: 19.8± 4.6, 1 males and 11 females) and 14 age and sex-matched healthy controls (age: 19.4± 4.9) were recruited. Parents of patients were asked to finish a questionnaire about the clinical presentation. The study was approved by the Institutional Review Board of NTU hospital and informed consent was obtained from all participants. Imaging: All of the scans were acquired using a Siemens Tim Trio 3T scanner with a 32-channel phased array coil. MR imaging was consist of sagittal T1-weighted and axial T2 fast spin-echo. DSI data used a twice-refocused balanced echo diffusion echo planar imaging sequence with following parameters: TE=130 ms, TR = 9600ms, slice thickness = 2.5mm, FOV = 200 x 200 mm^2, matrix size = 80 x 80, 56 slices. A total of 102 diffusion encoding gradients with bmax(4000 s/mm^2) were applied in a half sphere of the 3D q-space. Analysis: Whole brain tract-based automatic analysis reconstructed on the NTU-DSI-122 template was used to obtain a 2D connectogram for each DSI dataset.7 The connectogram provides generalized fractional anisotropy (GFA) profiles of 76 white matter tract bundles. The means GFA values of 76 tracts were calculated by two sample t-test to investigate the difference between controls and patients. Bonferroni correction was used to account for multiple comparisons.Results
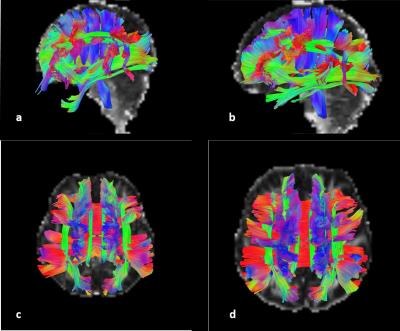
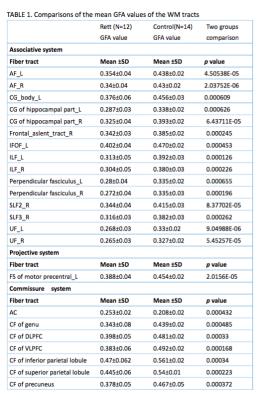
Most of patients lost their speech ability and purposeful hand use, and 8 of them can walk with abnormal gait. Comparing with healthy controls(Table1), GFAs in 23 of 76 tract bundles in RTT were significantly reduced after correction (p<0.0006), and most of them (15/23) were in associative system(Figure1), including bilateral arcuate fasciculus (AF), cingulum of hippocampus part, inferior longitudinal fasciculus(ILF), perpendicular fasciculus, and uncinated fasciculus(UF); left inferior frontal occipital fasciculus (IFOF), and cingulum of body part; right frontal aslent tract, superior longitudinal fasciculus II (SLF II), and SLF III. Left frontal striatum (FS) of motor precentral is the only tract with significantly lower GFA in projective systems, even though 6 of 8 striatum-connected tracts were reduced in RTT (p<0.05). The callosal fibers in commissure system were significantly reduced. No difference in all of the corticospinal fiber tracts (CST) with healthy controls was detected. In RTT, the value of GFA in anterior commissure (AC) showed higher than controls(p=0.0004).Discussion
As the first study with systematically analysis in younger adult of RTT, we tried to investigate the microstructural alterations of white matter tracts in their whole brain. In accordance with previous studies, lower diffusion indices were detected in corpus callosum, cingulate gyrus, IFOF and SLF.5,6 Interestingly, several alternations of microstructure, including AF8, UF9, perpendicular fasciculus, IFOF10 and frontal aslent tract11, were related to language function, and might be associated with the speech development of RTT. In the light of clinical observation, most RTT did not achieve early speech-language millstone on the pre-regression period.12 Patients usually present impairments of movement, however, CST of RTT were preserved and striatum-connected tracts were reduced. The reason might be the abnormality alterations in dopaminergic function in striatum.13,14 According to an eye tracking study, RTT showed the ability to process visual stimuli with preferential attention to novelty, in the same way with typical children.15 AC played an important role in visual attention and color perception and the significantly increasing in microstructural integrity supported the clinical phenomenon of visual perception. In summary, RTT in younger adults were found to have specific pathologic changes in microstructures of white matter, which will help us to get more clear understanding of RTT and common biomarkers.Acknowledgements
We thank members of ABMRI lab in NTU for their technical support in analyzing the diffusion spectrum imaging .References
[1]Bienvenu, T., Philippe, C., De Roux, N., et al.(2006). The incidence of Rett syndrome in France. Pediatric neurology, 34(5), 372-375.
[2]Chahrour, M., & Zoghbi, H. Y. (2007). The story of Rett syndrome: from clinic to neurobiology. Neuron, 56(3), 422-437.
[3]Armstrong, D. D. (2005). Neuropathology of Rett syndrome. Journal of child neurology, 20(9), 747-753.
[4] Carter, J. C., Lanham, D. C., Pham, D., et al. (2008). Selective cerebral volume reduction in Rett syndrome: a multiple-approach MR imaging study. American Journal of Neuroradiology, 29(3), 436-441.
[5]Izbudak, I., Farage, L., Bonekamp, D., et al. (2009). Diffusion tensor imaging findings in Rett syndrome patients. In Proceedings of the 17th ISMRM Scientific Meeting, Honolulu, HI.
[6]Mahmood, A., Bibat, G., Zhan, A. L., et al (2010). White matter impairment in Rett syndrome: diffusion tensor imaging study with clinical correlations. American Journal of Neuroradiology, 31(2), 295-299.
[7] Chen, Y. J., Lo, Y. C., Hsu, Y. C., et al. (2015). Automatic whole brain tract-based analysis using predefined tracts in a diffusion spectrum imaging template and an accurate registration strategy.Human brain mapping, 36(9), 3441-3458.
[8]Catani, M., & Mesulam, M. (2008). The arcuate fasciculus and the disconnection theme in language and aphasia: history and current state. Cortex, 44(8), 953-961.
[9]Duffau, H., Gatignol, P., Moritz-Gasser, S. , et al. (2009). Is the left uncinate fasciculus essential for language?. Journal of neurology, 256(3), 382-389.
[10]Mandonnet, E., Nouet, A., Gatignol, P., et al. (2007). Does the left inferior longitudinal fasciculus play a role in language? A brain stimulation study. Brain, 130(3), 623-629.
[11] Kronfeld-Duenias, V., Amir, O., Ezrati-Vinacour, R., et al .(2016). The frontal aslant tract underlies speech fluency in persistent developmental stuttering. Brain Structure and Function, 221(1), 365-381.
[12] Bartl-Pokorny, K. D., Marschik, P. B., Sigafoos, J., et al. (2013). Early socio-communicative forms and functions in typical Rett syndrome. Research in developmental disabilities, 34(10), 3133-3138.
[13] Jellinger, K., Seitelberger, F., Opitz, J. M., et al. (1986). Neuropathology of Rett syndrome. American Journal of Medical Genetics, 25(S1), 259-288.
[14] Wenk, G. L. (1995). Alterations in dopaminergic function in Rett syndrome. Neuropediatrics, 26(02), 123-125.
[15] Djukic, A., McDermott, M. V., Mavrommatis, K., et al. (2012). Rett syndrome: basic features of visual processing—a pilot study of eye-tracking. Pediatric neurology, 47(1), 25-29.
[16] Barr, M. S., & Corballis, M. C. (2002). The role of the anterior commissure in callosal agenesis. Neuropsychology, 16(4), 459.
Figures