2314
Comprehensive assessment of white matter alterations in Tourette syndrome using automatic whole-brain tract-specific analysis1Institute of Medical Device and Imaging, National Taiwan University College of Medicine, Taipei, Taiwan, 2Institute of Biomedical Engineering, National Taiwan University College of Medicine, Taipei, Taiwan, 3Department of Pediatrics, National Taiwan University College of Medicine, Taipei, Taiwan, 4Graduate Institute of Brain and Mind Sciences, National Taiwan University College of Medicine, Taipei, Taiwan, 5Department of Medical Imaging, National Taiwan University Hospital, Taipei, Taiwan, 6Molecular Imaging Center, National Taiwan University, Taipei, Taiwan
Synopsis
To identify microstructural alteration of white matter tracts in patients with Tourette syndrome (TS), diffusion spectrum imaging data were obtained from 14 patients and 14 matched controls. Whole-brain tract-based automatic analysis was employed to investigate the differences in white matter microstructures between the two groups. As compared with the controls, patients with TS showed altered tract integrity in callosal fibers, cingulum, thalamic radiations and corticospinal tracts. The altered white matter tracts account for clinical hallmarks and pathophysiology of TS, and might serve as structural correlates of TS.
Purpose
Tourette syndrome (TS)
is a neuropsychiatric disorder characterized by chronic motor and phonic tics
that afflicts more frequently in males than females (ratio: 3 to 1). Integrity
of the white matter tracts specific to motor and vocal tics should be altered.
To test this hypothesis, we performed tract-specific analysis over the whole brain to
measure the microstructural properties of 76 major white matter tracts in a
systematic way.
Method
Subjects: The subjects
consisted of 14 patients with clinical diagnosis of TS (gender: 12 males and 2
females, age: 9.79±2.75 years) and 14 age- and sex-matched healthy controls.
Imaging: MRI scans
were performed on a 3T MRI system (TIM Trio, Siemens, Erlangen) with a
32-channel phased array coil. T1-weighted imaging utilized a 3D magnetization-prepared
rapid gradient echo pulse sequence: TR/TE = 2000/3 ms, flip angle = 9。,
FOV = 256 × 192 × 208 mm^3, matrix size = 256 × 192 × 208, and spatial resolution
= 1 x 1 x 1 mm^3. Diffusion spectrum imaging (DSI) used a twice-refocused
balanced echo diffusion echo planar imaging sequence, TR/TE = 9600/130 ms, FOV =
200 x 200 mm^2, matrix size = 80 × 80, 56 slices, slice thickness = 2.5 mm and
a total of 102 diffusion encoding gradients with the maximum diffusion sensitivity
bmax = 4000 s/mm^2.
Analysis: Whole-brain tract-based automatic
analysis (TBAA) was performed to obtain generalized fractional anisotropy (GFA)
profiles of 76 fiber tracts. The procedure of TBAA method was described in our
previous study1. Two sample T-test was performed to investigate the
difference in mean GFA of each tract between patient and control groups. A
threshold free cluster weighted (TFCW) method was used following Smith’s
approach2 to estimate the weighted score of the
effect size for each step of the tract. The segments that were in the top 2
percentile of the weighted scores were selected as the segments of most
difference.Results
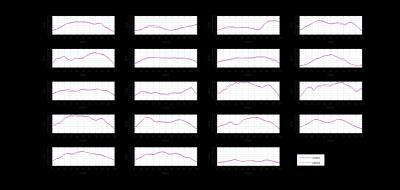
We found reduced mean GFA in the right cingulum (CG) of hippocampal component, right inferior longitudinal fasciculus (ILF), right corticospinal tract (CST) of mouth and left CST of toe in two sample T-test (p < 0.05, uncorrected). In the TFCW estimation, the segments of most difference were located in 19 fiber tracts including the left arcuate fasciculus (AF), bilateral CG of hippocampal component, left frontal aslant tract, left inferior frontal occipital fasciculus (IFOF), bilateral inferior longitudinal fasciculus (ILF), left perpendicular fasciculus, left stria terminalis, left CST of trunk, right CST of mouth, left CST of toe, left thalamic radiation (TR) of the dorsal lateral prefrontal cortex (DLPFC), left TR of the precentral gyrus, left TR of the postcentral gyrus, bilateral TR of the optic radiation, anterior commissure (AC) and callosal fiber (CF) of the superior temporal gyrus. Most of these segments showed reduced GFA values in patients; only segments in the left TR of the postcentral gyrus, left TR of the precentral gyrus and AC showed increased GFA. GFA profiles of these tracts were plotted in figure 1. These tracts were rendered on a T1 template (figure 2).Discussion
Previous studies found an apparent decrease in fractional anisotropy (FA) in patients with TS3,4. It might account for decreased inhibition from contralateral brain regions. In this study, we only found a portion of the CF, i.e. the CF of the superior temporal gyrus, showing decreased GFA in patients. This might be due to the small sample size or early stage of TS in our subjects. The cingulate gyrus has numerous interconnections with regions involved in tic generation5. So, altered GFA in the CG might account for abnormal functions of the cingulate gyrus in TS. Schultz and colleagues verified that children with TS performed significantly worse in the visual-motor integration skills than controls6. Our findings of the ILF and IFOF, which belong visual pathways, might account for this behavioral deficit. Previous studies have found increased FA in pre- and post-central gyrus in TS7,8. Consistently, our patients showed local GFA increase in the TR of the pre- and post-central gyrus near the cortex. This might account for tics-related hyperactivity or compensation secondary to gray matter thinning in these cortical areas9. Most notably, our results are mainly located in the CST and TR. This finding indicates that characteristic impairment of white matter tracts might serve as the structural correlates of TS.conclusion
We
have characterized white matter tract impairment in TS using an automatic whole-brain
tract-based analysis method. The altered white matter tracts could account for
the clinical hallmarks and pathophysiology of TS.Acknowledgements
No acknowledgement found.References
1. Chen, Y., Lo, Y., Hsu, Y., Fan, C., Hwang, T., Liu, C., Chien, Y., Hsieh, M., Liu, C., Hwu, H. and Tseng, W. (2015). Automatic whole brain tract-based analysis using predefined tracts in a diffusion spectrum imaging template and an accurate registration strategy. Human Brain Mapping, 36(9), pp.3441-3458.
2. SMITH, S. and NICHOLS, T. (2009). Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage, 44(1), pp.83-98.
3. Oestreich, A. (2008). Reduced white matter connectivity in the corpus callosum of children with Tourette syndrome. Yearbook of Diagnostic Radiology, 2008, pp.138-139.
4. Cavanna, A., Stecco, A., Rickards, H., Servo, S., Terazzi, E., Peterson, B., Robertson, M., Carriero, A. and Monaco, F. (2010). Corpus callosum abnormalities in Tourette syndrome: an MRI-DTI study of monozygotic twins. Journal of Neurology, Neurosurgery & Psychiatry, 81(5), pp.533-535.
5. Peterson, B., Staib, L., Scahill, L., Zhang, H., Anderson, C., Leckman, J., Cohen, D., Gore, J., Albert, J. and Webster, R. (2001). Regional Brain and Ventricular Volumes in Tourette Syndrome. Archives of General Psychiatry, 58(5), p.427.
6. Schultz, R., Carter, A., Gladstone, M., Scahill, L., Leckman, J., Peterson, B., Zhang, H., Cohen, D. and Pauls, D. (1998). Visual-motor integration functioning in children with Tourette syndrome. Neuropsychology, 12(1), pp.134-145.
7. Draganski, B., Martino, D., Cavanna, A., Hutton, C., Orth, M., Robertson, M., Critchley, H. and Frackowiak, R. (2010). Multispectral brain morphometry in Tourette syndrome persisting into adulthood. Brain, 133(12), pp.3661-3675.
8. Thomalla, G., Siebner, H., Jonas, M., Baumer, T., Biermann-Ruben, K., Hummel, F., Gerloff, C., Muller-Vahl, K., Schnitzler, A., Orth, M. and Munchau, A. (2009). Structural changes in the somatosensory system correlate with tic severity in Gilles de la Tourette syndrome. Brain, 132(3), pp.765-777.
9. Worbe, Y., Gerardin, E., Hartmann, A., Valabregue, R., Chupin, M., Tremblay, L., Vidailhet, M., Colliot, O. and Lehericy, S. (2010). Distinct structural changes underpin clinical phenotypes in patients with Gilles de la Tourette syndrome. Brain, 133(12), pp.3649-3660
Figures