2310
Understanding white matter pathology in ALS using a multimodal approach1Clinical Imaging Sciences Centre, Brighton and Sussex Medical School, Brighton, United Kingdom, 2Centre for Medical Image Computing and Computer Science, University College London, London, United Kingdom, 3Trafford Centre for Medical Research, Brighton and Sussex Medical School, Brighton, United Kingdom
Synopsis
Multimodal diffusion MRI and quantitative MT techniques were used to examine the nature of abnormalities in the corticospinal tracts (CSTs) of amyotrophic lateral sclerosis (ALS) patients. Our data show reductions in axonal volume fraction (AVF) located superiorly to reductions in myelin volume fraction (MVF). We also find extensive decreases in fiber volume fraction (FVF) throughout the entire CST, supporting the hypothesis of axonal loss as the primary pathological mechanism in ALS.
Background
Quantitative MRI studies have consistently shown changes or abnormalities in the corticospinal tract (CST) of patients with amyotrophic lateral sclerosis (ALS). However, the nature of these changes is not fully understood; in particular, it is unclear whether these changes include demyelination, axonal loss or axonal degeneration.
Recently, a framework has been introduced which allows the estimation of the axonal volume fraction (AVF), myelin volume fraction (MVF) and the average voxel g-ratio, i.e. the ratio of the inner to the outer diameter of a myelinated axon [1].
The aim of this work is to assess whether abnormalities in the CST in ALS patients are predominantly due to changes in AVF and/or MVF, and if so, whether these translate into changes to the g-ratio, which could account for some of the disability observed.
Methods
Data from 21 ALS patients (M/F=15/6, mean age=64.5 years (SD 9.23), range: 45-73 years) and 19 healthy controls (M/F=12/7, mean age=60.9 years (SD 8.24), range: 43-76 years) were acquired on a 1.5T MRI scanner, including multi-shell diffusion-weighted data (10 b=0 volumes, 9 directions with b=300 smm-2, 30 directions with b=800 smm-2, and 60 diffusion directions with b=2400 smm-2), optimised for neurite orientation dispersion and density imaging (NODDI, [2]), and a quantitative MT protocol based on balanced steady-state free precession (bSSFP), as described in [3]. A T1-mapping sequence was also acquired. Total acquisition time was approximately half an hour. Quantitative MT data were analysed using in-house software, yielding a voxel-wise estimation of the macromolecular pool size ratio, F. dMRI data were analysed using the NODDI toolbox [2], to yield maps of the volume of the intra-cellular water compartment (Vic) and the isotropic component (Viso). Quantitative MT and NODDI data were non-linearly co-registered using the Advanced Normalization Tools (ANTs) 2.1.0. AVF, MVF and g-ratio maps were computed as previously described [4], then warped into MNI space. Fiber volume fraction (FVF=AVF+MVF) maps were also obtained. The JHU white-matter tractography atlas, available with FSL, was then used to extract an unbiased mask of the CST (thresholded at 25% and binarized).
Permutation inference [5] was performed on the masked images with randomise, available with FSL, using the Threshold-Free Cluster Enhancement (TFCE) method. Age was included as a covariate, and results were accepted as significant for p < 0.05 after correction for multiple comparisons.
Results
FVF was extensively reduced throughout the CST, bilaterally (Fig 1). In order to establish whether these abnormalities were primarily due to myelin or axonal loss, we looked at changes in AVF and MVF separately. Decreases in both AVF and MVF were detected in patients. The changes in AVF were primarily located in the superior aspect of the CST, while the changes in MVF were primarily located in the inferior aspect of the CST. No significant changes were detected in the g-ratio.Discussion
Our data confirm extensive degeneration of the CSTs in patients with ALS. The respective location of AVF and MVF decreases may indicate predominantly dying-back mechanisms, although these data are not conclusive. In general, the substantial decrease in FVF supports the hypothesis of axonal loss as the primary pathological mechanisms in ALS.Acknowledgements
MC Gabel is funded by the Motor Neurone Disease Association.References
1) Stikov et al. In vivo histology of the myelin g-ratio with magnetic resonance imaging. Neuroimage 2015, 4:368-73
2) Zhang H, et al. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage. 2012, 61:1000-16
3) Gloor et al., Quantitative magnetization transfer imaging using balanced SSFP. Magn Reson Med. 2008, 60:691-700
4) Cercignani et al., Mapping the g-ratio within MS lesions. Proc ISMRM 2015, # 1402
5) Winkler et al., Permutation inference for the general linear model, NeuroImage, 2014, 92:381-397
Figures
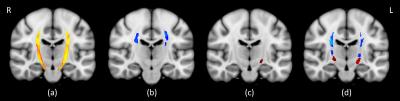
Reductions in FVF (a), AVF (b) and MVF (c) were found in the CSTs of patients compared with controls. Results were accepted as significant for p < 0.05, corrected for multiple comparisons using the Threshold-Free Cluster Enhancement (TFCE) method.
Panel (d) shows results for AVF (blue) and MVF (red) at a less stringent threshold of p < 0.1; here the bilateral distribution is clearly visible, as is the superior/inferior divide between AVF and MVF reductions.