2304
Microstructural heterogeneity of Superior longitudinal fasciculus (SLF-II) predicts impulsivity in healthy young girls1Harvard Medical School, Boston, MA, United States, 2Brigham and Women's Hospital, Boston, MA, United States
Synopsis
Diffusion MRI (dMRI) is sensitive to microstructural arrangement of cells and axons in the brain. In this abstract, we analyzed dMRI data from 30 healthy children (14 girls, 13.9 ± 0.8 yrs, 16 boys, 13.4 ± 0.9 yrs). Advanced multi-tensor tractography was used to trace the SLF-II and heterogeneity in fractional anisotropy (HFA)4 (the standard deviation in FA) was computed in all subjects. Subjects were separated into two groups (males, females) and correlation between HFA and total Barratt Impulsivity Scores (BIS) was computed for each group. A statistically significant correlation was found between HFA in left SLF-II and BIS in girls but not in boys. The SLF-II has been known to be involved in spatial attention and executive control and higher heterogeneity in white matter integrity in SLF-II seems to be involved in impulsive/attentional network in young girls but not boys.
Purpose
Age-related improvements in higher-order cognitive domains, such as executive functioning, are thought to be related not only to a marked reorganization of the frontal lobe, but also to improved functional white matter (WM) connectivity within and between brain regions during adolescence. Adolescence also has been characterized as a time of increased propensity to seek out novel stimulation and to engage in risk-taking or impulsive behavior. Impulsiveness is a behavioral trait that also has been observed in a variety of conditions and behaviors, including attention deficit disorder (ADHD), alcohol and substance use disorders, binge-eating disorders and gambling. Thus, the rapid development of the prefrontal cortex, including increased WM connectivity between brain regions, may serve as a neurobiological mechanism underlying impulsiveness during adolescence. There is increasing evidence that behavioral and neurobiological milestones related to adolescent brain development occur in a sex-specific manner5, which may therefore confer a differential set of unique biomarkers of risk taking for girls compared to boys.Methods.
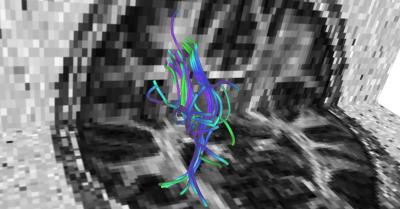
Data acquisition and preprocessing: Thirty adolescents, recruited from the local community, served as participants in this study. Imaging data were acquired on a 3T Siemens TIM Trio scanner, which included a T1-weighted, T2-weighted and a diffusion MRI scan. The spatial resolution for T1-weighted images was 1mm3, while for the dMRI data it was 1.9mm x 1.9mm x 3.75mm. For the dMRI data, there were a total of 72 gradient directions at a b-value of 1000 s/mm2 and 8 non-diffusion weighted images. Semi-automated quality control was done using in-house scripts and all gradient directions with signal drop were removed. Eddy current and head motion correction was done using FSL tools. FreeSurfer software1 was used to parcellate the brain from the T1-weidhted image. The parcellations were then transformed to the dMRI space using the ANTS non-rigid registration software. Whole brain tractography was run using the open-source unscented Kalman filter based multi-tensor tractography algorithm2 with 5 seeds per voxel and a stopping criteria of FA < 0.15 and generalized FA < 0.1 (all default parameters). The white matter query language (WMQL)3 was used to extract out the left and right SLF-II (see Figure 2) in all subjects as it is involved in spatial attention and executive control. Subsequently, heterogeneity in FA (HFA)4, which is the standard deviation of FA in a fiber bundle, was computed which captures the microstructural tissue heterogeneity in the fiber bundle.Results.
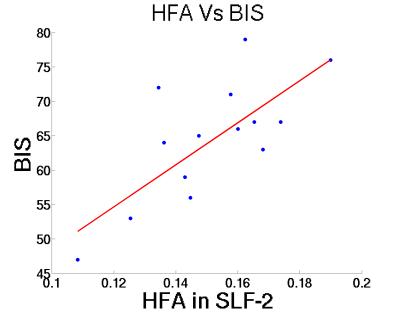
Data were analyzed for sex differences by examining correlations between HFA (left and right hemispheres) and total BIS scores, computed separately for males and females. Figure 1 shows the correlation between HFA of SLF-II and BIS scores. A high correlation of 0.72 with p-value = 0.0033 was evident in girls but not boys. Further, this statistically significant correlation was observed only on for the left hemisphere and only a trend for the right hemisphere.Discussion.
The SLF-II originates in the caudal-inferior parietal cortex and terminates in the dorsolateral prefrontal cortex (Brodmann area 6, 8 and 46). This pathway has been implicated in disorders such as ADHD, characterized by high impulsivity. While our sample involves healthy young subjects, the data indicate a potential neurodevelopmental risk factor for impulsiveness, which in adolescents can have negative consequences. Limitations of this study include a small sample size and relatively low-resolution diffusion MRI data. Nonetheless, these results provide an important perspective on adolescent brain development and associations with impulsiveness, a behavior intrinsic to the passage through the period of adolescence, which need to be confirmed in a larger sample size.Acknowledgements
Funding information: R01MH097979References
1. B. Fischl. FreeSurfer. Neuroimage. 2012;62(2):774-781.
2. J. G. Malcolm, M. E. Shenton, Y. Rathi.?Filtered multitensor tractography. IEEE Trans. Med. Imaging. 2010;29(9):1664-1675.
3. Wassermann, Demian, et al. "On describing human white matter anatomy: the white matter query language." International Conference on Medical Image Computing and Computer-Assisted Intervention. Springer Berlin Heidelberg, 2013.
4. Y. Rathi, O. Pasternak, P. Savadjiev, O. Michailovich, S. Bouix, M. Kubicki, C-F. Westin, N. Makris, and M. E. Shenton. "Gray matter alterations in early aging: A diffusion magnetic resonance imaging study." Human brain mapping 35, no. 8 (2014): 3841-3856.
5. Silveri MM, Rohan ML, Pimentel PJ, Gruber SA, Rosso IM, Yurgelun-Todd DA. Sex differences in the relationship between white matter microstructure and impulsivity in adolescents. Magnetic Resonance Imaging, 2006; 24(7):833-41. PMID: 16916700