2296
White Matter Abnormalities in Congenital Heart Disease Assessed Using Advanced Diffusion Imaging1Brigham and Women's Hospital, Harvard Medical School, Boston, MA, United States, 2Boston Children's Hospital, Harvard Medical School, Boston, MA, United States, 3VA Healthcare, Boston, MA, United States
Synopsis
The study examined white matter (WM) abnormalities in neonates with Congenital Heart Disease (CHD) (19 CHD and 16 typically developing (TD) neonates). Gaussian mixture model showed that neonates with CHD have lower cellular volume and density in UF, SFOF, left IFOF, and CC. Fractional Anisotropy was lower in neonates with CHD in bilateral UF, CC, and left SLF. NODDI results indicated lower intracellular volume in the bilateral SFOF, and higher fiber orientation dispersion in the left CC and SLF in CHD. Our results demonstrate that significant WM abnormalities related to language areas are seen in neonates with CHD.
Purpose
Congenital heart disease (CHD) affects nearly 1% of neonates1. Past studies show that neonates with CHD are prone to abnormal or delayed brain development, which leads to impairments ranging from motor and visual-spatial to cognitive dysfunctions2-5. In this study, we compare measures derived from three different diffusion MRI methods: Diffusion Tensor Imaging (DTI)6, Neurite Orientation Dispersion and Density Imaging (NODDI)7, and a non-parametric Gaussian Mixture Model (GMM)8 to elucidate the specific nature of microstructural abnormalities in several White Matter (WM) structures of neonates with CHD. We use fractional anisotropy (FA) obtained from DTI, orientation dispersion index (ODI), intra-cellular volume fraction (FICVF), and isotropic volume fraction (FISO) obtained from NODDI and return-to-origin probability (RTOP) and return-to-axis-probability (RTAP), measures sensitive to total cellular volume and axon density, respectively, derived from GMM. Thus far, DTI studies have only measured normal WM development and abnormalities associated with CHD. The purpose of this study is to examine the efficacy and sensitivity of all three methods for detecting developmental abnormalities in WM structures (11 regions) at early postnatal (39 to 47 weeks) in children with CHD.Method
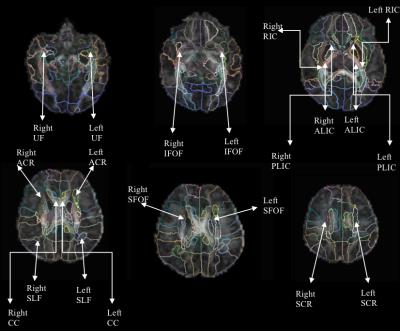
The sample included 19 CHD (mean age: 39.54 weeks, SD=1.08 weeks) and 16 typically developing (TD) neonates (mean age: 42.2 weeks, SD=2.28 weeks). MR acquisition was done on a 3T TimTrio Siemens scanner using a 32-channel head coil. A multi-b-value DWI protocol at b={1000, 2000} s/mm^2, with each shell having 30 gradient directions. Other acquisition parameters were: TR=3700 ms, TE=104 ms, and 2 mm isotropic spatial resolution, with a multi-slice acquisition lasting 6 minutes. All images were quality checked for signal drop, and were corrected for motion and eddy current correction using FSL flirt9,10. Diffusion tensors were fitted in each voxel to calculate FA6. RTOP and RTAP were estimated from full probability distribution of water displacement in brain tissue8,11,12. ODI, FISO, and FICVF were estimated using publicly available software using NODDI model7. Analysis: All images were up-sampled linearly to an isotropic voxel size of 0.6mm to match the resolution of John Hopkins Neonate (JHN) atlas. Affine and deformable transformations were used to register the JHN atlas to each subjects FA using Advanced Normalization Tools (ANTs)13. Mean values for each of the 6 measures (above) were computed for each subject from the 11 ROIs. Robust linear regression was used to regress out age related brain changes. The residuals from this model were used to compute Welch t-test to determine statistical differences between WM areas of CHD and TD neonates. FDR was used to correct for multiple comparisons.Results
Statistically significant results were:
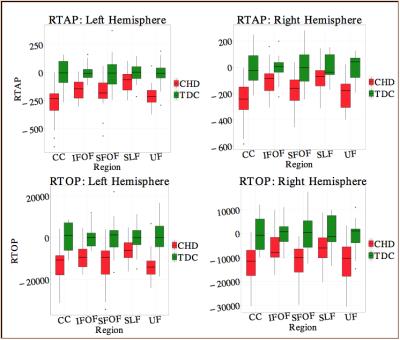
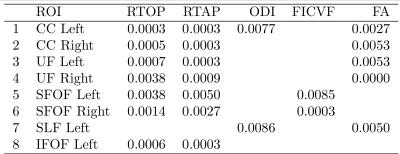
1. RTOP and RTAP were lower in CHD in: a) Bilateral uncinate fasiculus (UF), b) left Inferior Fronto Occipital Fasiculus (IFOF), c) bilateral Superior Fronto-Occipital Fasiculus (SFOF) and d) bilateral corpus callosum (CC) (Fig. 2) (P<0.01). These results indicate potentially lower axonal density and volume in CHD babies compared to controls.
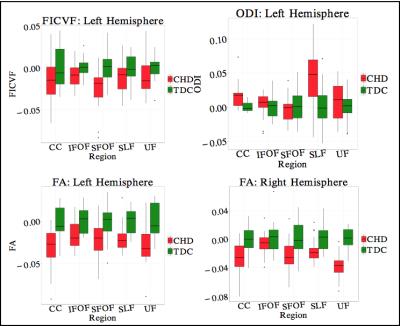
2. FA was low in CHD in: a) Bilateral UF, b) bilateral CC, and c) left SLF(p<0.01). While FA is not specific to a particular neuropathology, it is sensitive to white matter integrity in general, which seems to be affected in CHD.
3. ODI was higher in CHD in: a) left CC and left SLF, while b) FICVF was lower in CHD neonates in the SFOF (fig. 3) (P<0.01). These results indicate lower intracellular volume in SFOF in CHD along with higher fiber dispersion in CC and SLF.
Discussion
Results demonstrate slower maturation of WM structures in UF, SFOF, left IFOF, and CC among neonates with CHD. UF and IFOF are part of the ventral language pathway, which is important for semantic comprehension of language14. In addition, abnormalities in CC have been linked with several developmental disorders including developmental language delay15. These WM abnormalities may therefore be related to language deficits faced by CHD neonates in later stages of their lives. In terms of the methods used, it is clear from Figures 3 and 4, that the GMM method is more sensitive (and more specific) to pathologies in CHD, e.g., more regions are different and p-value between groups is 10 times smaller compared to the NODDI model.Conclusion
Our results demonstrate that significant WM abnormalities related to the language network are seen in neonates with CHD. Furthermore, using specific measures such as RTOP and RTAP, we can infer that CHD is characterized likely by lower axonal (and/or cellular) packing and density in bilateral CC, UF, SFOF and left IFOF. This result is crucial for understanding impairments associated with language development in CHD neonates.Acknowledgements
Funding information : R01MH097979References
1. Owen M, Shevell M, Majnemer A, Limperopoulos C. Abnormal brain structure and function in newborns with complex congenital heart defects before open heart surgery: a review of the evidence. - PubMed - NCBI. Journal of Child Neurology. 2011;26(6):743-755. doi:10.1177/0883073811402073.
2. Bellinger DC, Wypij D, Kuban KCK, et al. Developmental and Neurological Status of Children at 4 Years of Age After Heart Surgery With Hypothermic Circulatory Arrest or Low-Flow Cardiopulmonary Bypass. Circulation. 1999;100(5):526-532. doi:10.1161/01.CIR.100.5.526.
3. Hovels-Gurich HH. Long term behavioural outcome after neonatal arterial switch operation for transposition of the great arteries. Archives of Disease in Childhood. 2002;87(6):506-510. doi:10.1136/adc.87.6.506.
4. Bellinger DC, Wypij D, duPlessis AJ, et al. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: The Boston Circulatory Arrest Trial. The Journal of Thoracic and Cardiovascular Surgery. 2003;126(5):1385-1396. doi:10.1016/S0022-5223(03)00711-6.
5. Limperopoulos C, Majnemer A, Shevell MI, Rosenblatt B, Rohlicek C, Tchervenkov C. Neurologic Status of Newborns With Congenital Heart Defects Before Open Heart Surgery. Pediatrics. 1999;103(2):402-408.
6. Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. - PubMed - NCBI. Biophysical Journal. 1994;66(1):259-267. doi:10.1016/S0006-3495(94)80775-1.
7. Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC. NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain. NeuroImage. 2012;61(4):1000-1016. doi:10.1016/j.neuroimage.2012.03.072.
8. Ning L, Westin C-F, Rathi Y. Estimating diffusion propagator and its moments using directional radial basis functions. IEEE transactions on medical imaging. 2015;34(10):2058-2078. doi:10.1109/TMI.2015.2418674.
9. Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5(2):143-156.
10. Jenkinson M, Bannister P, Brady M, Smith S. Improved Optimization for the Robust and Accurate Linear Registration and Motion Correction of Brain Images. NeuroImage. 2002;17(2):825-841. doi:10.1006/nimg.2002.1132.
11. Cheng J. Estimation and Processing of Ensemble Average Propagator and Its Features in Diffusion MRI. May 2012.
12. Özarslan E, Koay CG, Shepherd TM, et al. Mean apparent propagator (MAP) MRI: A novel diffusion imaging method for mapping tissue microstructure. NeuroImage. 2013;78:16-32. doi:10.1016/j.neuroimage.2013.04.016.
13. Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage. 2011;54(3):2033-2044. doi:10.1016/j.neuroimage.2010.09.025.
14. Chang EF, Raygor KP, Berger MS. Contemporary model of language organization: an overview for neurosurgeons. Journal of Neurosurgery. 2015;122(2):250-261. doi:10.3171/2014.10.JNS132647.
15. Paul LK. Developmental malformation of the corpus callosum: a review of typical callosal development and examples of developmental disorders with callosal involvement. J Neurodevelop Disord. 2010;3(1):3-27. doi:10.1007/s11689-010-9059-y.
Figures