2294
Comparison of postnatal trajectory of neonatal white matter development between preterm and term neonates during the neonatal stage1Department of Diagnostic Radiology, the first Affiliated Hospital of Xi'an Jiaotong University, Xi'an, People's Republic of China, 2Department of Biomedical Engineering, School of Life Science and Technology, Xi'an Jiaotong University, Xi'an, People's Republic of China, 3MR Research China, GE Healthcare, Beijing, People's Republic of China
Synopsis
Due to exposure to extrauterine environment, neonatal brain development is in totally different ways after birth, compared with the ‘protected’ gestation. However, little is known about the postnatal trajectory of neonatal brain development, especially for the differences between preterm and term neonates. This study aims to investigate the postnatal maturation of brain white matter (WM) during the neonatal stage and further provide comparison between preterm and term neonates. Our results suggest that during the neonatal stage, preterm neonates show weaker development capacity than term in optical and somatosensory functions, while present catching up maturation in motor function.
Introduction
From birth, exposure to extrauterine environment makes the neonatal brain develop in totally different ways from the “protected” gestation1. Previous studies demonstrated preterm neonates have higher risk in neurodevelopmental delay/impairment than terms2. Besides the prematurity, stimulation from extra-uterine environment also enhances the brain development3. However, little was known about the postnatal trajectory of neonatal brain development, especially the differences between preterm and term neonates during the neonatal stage. Therefore, this study aims to investigate the postnatal maturation of brain white matter (WM) as indicated by the effects of postnatal age on fractal anisotropy (FA) of WM and further provide comparison between preterm and term neonates.Methods
The Institutional Review Board approved this study and all the written informed consents were obtained from parents of neonates. Patients 118 neonates with no abnormality on MRI were included. MR Protocols. All MR examinations were performed using a 3T scanner (Signa HDxt, GE Healthcare, Milwaukee, Wisconsin) with an 8-channel head coil. The protocols included sequences: (1) a transverse 3D T1-weighted sequence (TR/TE, 10ms/4.6ms; matrix, 256×256; section-thickness, 1mm; FOV, 240mm); (2) a 2D T2-weighted sequence (TR/TE, 4200ms/120ms; matrix, 256×256; section-thickness, 4mm; FOV, 180mm); (3) Diffusion tensor imaging (DTI; 35 directions; b-value, 1000 s/mm2; TR/TE, 5500ms/95ms; section-thickness, 4mm; FOV, 180mm, matrix, 128×128). Data and statistical analysis DTI data was processed with the aid of the FMRIB software library (FSL, www.fmrib.ox.au.uk/fsl). The TBSS and scatterplots with linear fitting were used to investigate relations between postnatal age on DTI-derived FA values respectively for the preterm and term neonates. Gestational age (GA) was considered as covariate. Here, the typical visual, auditory and sensorimotor WMs, i.e. OR (optical radiation), AR (auditory radiation), CST (corticospinal tract) and thal-PSC (thalamus-primary somatosensory cortex) were selected as ROIs based on the Johns Hopkins WM label atlas.
All statistical analysis were performed by using SPSS 17.0 (SPSS, Chicago, IL, USA); p<0.05 was considered as statistically significant difference.
Results
Among 118 neonates, 50 preterm (male/female, 28/22; GA range, 30~37weeks) and 68 term (male/female, 42/26; GA range, 37~42weeks) were included. Significant differences presented in postnatal age and GA between preterm and term, while no differences were observed in terms of gender, and 10min-APGAR (Table 1).
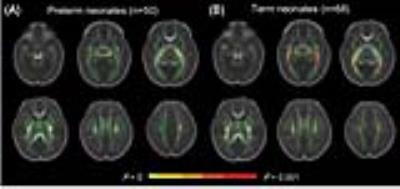
Postnatal age (day 1~28) of preterm neonates showed significant correlations with FA values in regional WM, e.g. corpus callosum, anterior limb of internal capsule centrum and etc. (p<0.001); while, no relations were observed for postnatal age range of day 1~14. Similar findings were observed in term neonates. Specifically, significant correlations were observed in different WM regions, such as centrum semiovale, brainstem, posterior limb of internal capsule, corticospinal tract and etc. (p<0.001). (Figure 1)
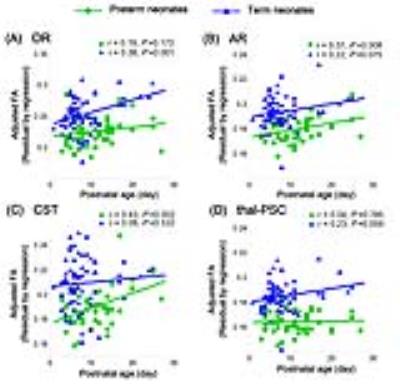
For OR and thal-PSC, term neonates showed higher linear relation between adjusted FA values and postnatal age than preterm (OR: rpreterm=0.19, p=0.173, rterm=0.38, p=0.001; thal-PSC: rpreterm=0.19, p=0.173, rterm=0.38, p=0.001). While for AR and CST, preterm neonates presented higher linear relation than term (AR: rpreterm=0.37, p=0.008, rterm=0.22, p=0.075; CST: rpreterm=0.43, p=0.002, rterm=0.08, p=0.532). (Figure 2)
Discussion
This study indicated that postnatal WM development during neonatal stage presented varying maturation characteristics due to the increasing effects of external stimuli. In comparison to term neonates, preterm showed weaker postnatal growth of regional white matter, e.g. OR and thal-PSC.
Using TBSS, significant correlations of FA with postnatal age in preterm neonates mainly presented in motor-associated WM regions; while term neonates presented more WM regions. Through scatterplots with linear fitting, higher linear correlation between adjusted FA and postnatal age in OR and thal-PSC may hint the weaker capacity of postnatal WM maturation of preterm than term neonates, e.g. perception ability of extra-uterine stimuli4. For AR, preterm and term presented consistently increasing growth. The obviously growth of FA values in preterm CST may suggest the catching up maturation. It may be the postnatal motor stimulation that accelerate the CST maturation of preterm.
Conclusion
During the neonatal stage, preterm neonates show weaker development capacity than term in optical and somatosensory functions, while present catching up maturation in motor function.Acknowledgements
This work was supported by the National Key Research and Development Program of China (2016YFC0100300), National Natural Science Foundation of China (No.81171317, 81471631), and the 2011 New Century Excellent Talent Support Plan of the Ministry of Education, China (NCET-11-0438).References
1. Dubois J, Dehaene-Lambertz G, Kulikova S, et al. The early development of brain white matter: a review of imaging studies in fetuses, newborns and infants. Neuroscience, 2014, 276: 48-71.
2. Qiu A, Mori S, Miller MI. Diffusion tensor imaging for understanding brain development in early life. Annu Rev Psychol, 2015, 66: 853-876.
3. Dubois J, Dehaene-Lambertz G, Kulikova S, et al. The early development of brain white matter: a review of imaging studies in fetuses, newborns and infants. Neuroscience, 2014, 276: 48-71.
4. Groppo M, Ricci D, Bassi L, et al. Development of the optic radiations and visual function after premature birth. Cortex, 2014, 56: 30-37.
Figures