2290
Structural thalamocortical connectivity in the developing infant brain1Centre for the Developing Brain, King's College London, London, United Kingdom, 2Institute of Psychiatry, Psychology and Neuroscience, King's College London, London, United Kingdom
Synopsis
Thalamocortical development in early life is crucial for normal brain functioning and abnormalities to these networks are thought to underpin atypical neurodevelopment. However, to date examination of this system in the infant has been hampered by the lack of age-appropriate population atlases. In this study we circumvent this problem by applying independent component analysis to parcellate the thalamocortical projections and their underlying thalamic seed in 6-months-old infants using diffusion MRI.
Introduction
Thalamocortical networks play a crucial role in the development and maturation of numerous cognitive and sensorimotor brain circuits.1 Abnormalities in the structure and function of these networks are thought to underpin atypical development,2 including conditions such as autism spectrum disorder (ASD).3 ASD is diagnosed in early childhood, however, emerging evidence suggests that abnormal connectivity is evident before symptom presentation in the first year of life.4 A better understanding of the thalamocortical development at this earlier age is therefore crucial, not only for understanding normal neurodevelopment but also for identifying the underlying mechanisms of ASD. Studying the thalamocortical connectivity in early infancy, however has been challenging due to rapidly changing brain tissue properties and lack of age-appropriate population atlases. The aim of this study is to apply connectivity-based parcellation of the thalamocortical fibres and their underlying seed region using independent component analysis (ICA) in the developing infant brain.Methods
Acquisition. 32 infants (male/female=15/17) underwent MRI at 6 months (M=188 days; SD=10 days) on a 3T Philips system. Infants were sedated with oral chloral hydrate (50-80 mg/kg) prior to scanning and were monitored throughout. Image acquisition included T1 MPRAGE anatomical images with voxel resolution of 1.1x1.1x2mm3 and high angular resolution diffusion imaging (HARDI) in 64 non-collinear directions with b-value of 2500 s/mm2 , 4 non-diffusion weighted (b=0) volumes, and voxel size 2x2x2mm3, SENSE factor 2.
Data processing. A study-specific T1 template was created using 8 infants from the sample. The infants were selected based on data quality (minimal subject motion and artefacts), age at scan (M=188 days; SD=2) and gender (male/female=4/4). The T1 images were bias-field corrected, normalised, rigid registered to an initial mean target and an unbiased template image was calculated using ANTs.5 The resulting T1 template was used as a study-specific “standard” space. The bilateral thalami were manually segmented according to previously described anatomical borders6 and used as seed regions for tractography.
HARDI data were corrected for subject motion and eddy currents in FSL.7 BedpostX was used to prepare the data for probabilistic tractography, modelling up to 3 fibre populations per voxel. The diffusion data was non-linearly registered to the structural template space. Tractography was performed in ProbtrackX where 5000 streamlines were followed from each voxel of the left/right thalamus seed mask. To focus only on the ipsilateral thalamocortical connections, exclusion masks were set at the midline and at the brainstem. This resulted in 3D volume “tractograms” representing the spatial connectivity of each seed voxel, which were later concatenated into two 4D volumes per subject, representing all tractograms for the left and right thalamus respectively. Tractography was performed in native diffusion space but output in T1 template space with a 2mm isotropic voxel size.
Tractograms parcellation. Independent component analysis (ICA) is a blind source separation technique that enables the decomposition of spatially independent components and does not carry the inherent bias of region-of-interest approaches.8,9 The tensorial extension of ICA as implemented in MELODIC FSL was used to parcellate the thalamocortical bundles and their thalamic seed region. The dimensionality of the decomposition was set to 10 per hemisphere.
Mean fractional anisotropy (FA) and mean diffusivity (MD) were extracted from each component. Analyses were carried out in R software (www.r-project.org) and p-values were adjusted for multiple testing using false discovery rate (FDR).
Results
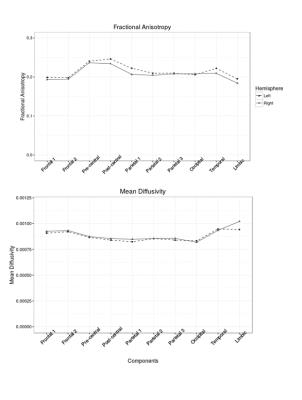
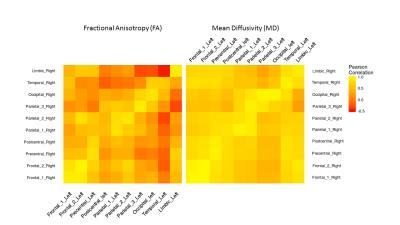
The estimated components were classified according to their cortical/subcortical projection. These included frontal, precentral, postcentral, parietal, occipital, temporal cortex and limbic system. There was a symmetrical spatial distribution of each component and its underlying thalamic origin in the left and right hemisphere (Figure 1). The extracted mean FA/MD showed a similar pattern across hemisphere (no significant difference between left and right components, Figure 2) with high positive correlation (p < 0.05, adjusted for FDR) in diffusivity values between the same component in the left and the right hemisphere (Figure 3).Discussion and Conclusion
We demonstrate the effectiveness of applying ICA to segment thalamocortical fibre bundles and their underlying seed region in the developing infant brain. Our analyses showed a symmetrical left/right spatial distribution both for the thalamocortical projection and their thalamic seed of origin. The application of this data-driven approach to infant data carries the potential to advance our knowledge of typical and atypical development in early life as it does not rely on population atlases, not yet available for this age.Acknowledgements
No acknowledgement found.References
1. Poh, J. S., et al. "Developmental synchrony of thalamocortical circuits in the neonatal brain." NeuroImage 116 (2015): 168-176.
2. Ball, G., et al. "Thalamocortical connectivity predicts cognition in children born preterm." Cerebral Cortex 25.11 (2015): 4310-4318.
3. Nair, A., et al. "Impaired thalamocortical connectivity in autism spectrum disorder: a study of functional and anatomical connectivity." Brain 136.6 (2013): 1942-1955.
4. Wolff, J.J., et al. "Differences in white matter fiber tract development present from 6 to 24 months in infants with autism." American Journal of Psychiatry 169.6 (2012): 589-600.
5. Avants, B.B., et al. "A reproducible evaluation of ANTs similarity metric performance in brain image registration." Neuroimage 54.3 (2011): 2033-2044.
6. Srinivasan, L., et al. "Quantification of deep gray matter in preterm infants at term-equivalent age using manual volumetry of 3-tesla magnetic resonance images." Pediatrics 119.4 (2007): 759-765
7. Smith, S. M., et al. "Advances in functional and structural MR image analysis and implementation as FSL." Neuroimage 23 (2004): S208-S219.
8. O'Muircheartaigh, J., et al. "Clustering probabilistic tractograms using independent component analysis applied to the thalamus." Neuroimage 54.3 (2011): 2020-2032.
9. Catani, M., et al. "Virtual in vivo interactive dissection of white matter fasciculi in the human brain." Neuroimage 17.1 (2002): 77-94.
Figures