2283
Precise mapping of the developing somatosensory homunculus in the preterm human brain with fMRI and robotic tools1Bioengineering, Imperial College London, London, United Kingdom, 2Centre for the Developing Brain, Kings College London, London, United Kingdom
Synopsis
The mature somatosensory cortex is known to be somatotopically organized, but it is not known when this functional organization emerges in human life. We aimed to map functional responses across the somatosensory cortex of preterm infants using fMRI and automated robotic tools. A preterm “homunculus” topology was identified with a spatially distinct distribution of functional responses following somatosensory stimulation delivered to the mouth, wrists and ankles. The results suggest that as seen in animal studies, the human preterm period is likely to be critical for the development of the somatosensory system.
Target audience
This research will interest researchers investigating neonatal brain function and development.Background and aims
In the mature brain, functional activity within the primary motor and somatosensory cortices characteristically maps to a “homunculus” topology. However, it is not clear when this somatotopic organization emerges in early human life. Small animal studies suggest that it may form across the equivalent period to the third trimester of human gestation and that the map can be permanently affected by adverse environmental events1,2. The human preterm period may therefore represent a critical window of vulnerability for the establishment of this organization, which may explain the high prevalence of cerebral palsy in this population. We therefore aimed to study if the somatosensory cortex during the preterm period is already organized into a somatotopic “homunculus” using fMRI and a set of previously optimized MR-compatible robotic tools3,4.Methods
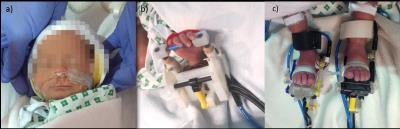
We studied 10 preterm infants (median age: 34+5 weeks PMA, range: 33+6 to 36+3 weeks PMA) using a 3-Tesla Philips MRI scanner (Achieva, Best NL), located at the Neonatal Intensive Care Unit at St Thomas Hospital, London. The study was approved by the NHS research ethics committee, and written consent was taken from all parents prior to the data acquisition. Infants with focal brain injury and/or diagnosed congenital brain abnormalities were excluded from the study group. All infants were studied during natural sleep, and physiological parameters were monitored throughout the acquisition. BOLD contrast fMRI images were acquired with a 32 channel head coil and an EPI sequence using the following parameters: TR/TE/FA = 1500msec/45msec/90o; resolution (x/y/z) = 2.5/2.5/3.25mm; 22 slices; total 256 volumes; 6 minutes and 34 seconds duration. Somatosensory cortical activation was induced using a set of custom-made, MR compatible and fully automated robotic tools. The devices were fitted to the infants’ limbs and head prior to the scan acquisition and were pneumatically actuated from the control room. Ankle and wrist devices provided a precise pattern of flexion and extension to the left and right ankle joints, and the left wrist joint (frequency 0.3Hz), while clinical use nasal cannula were repurposed to deliver a gentle puff of air toward the lips (0.4 atm at 0.3 Hz) (figure 1). Data from right wrist stimulation was taken from a previous study5. The task-fMRI consisted of a block design of 24 seconds of somatosensory stimulation alternating with 24 seconds of rest. Synchronisation between stimulation and image acquisition was ensured via detection of the scanner TTL pulse with a Data Acquisition card (Labview, National Instruments, Austin, TX, USA). Data was analysed using FSL (www.fmrib.ox.ac.uk/fsl). Individual subject statistical maps were created using a general linear model (GLM) as implemented in FEAT and an age-specific Hemodynamic Response Function4. Lower level functional activation maps were then registered to a preterm-specific template brain using non-linear registration, and a group analysis was performed for each of the stimulated body areas using a one-sample non-parametric t-test with permutation methods and threshold free cluster enhancement (family wise error corrected) as implemented in Randomise (v2.0).Results
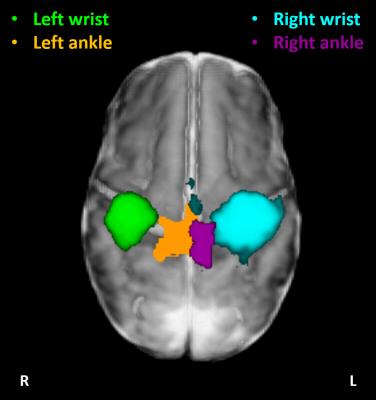
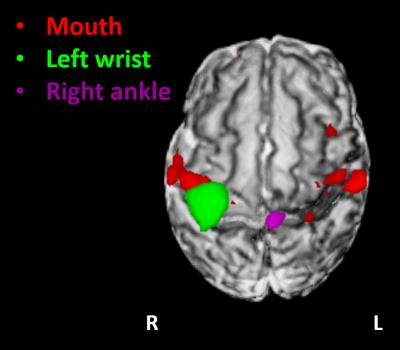
Data with excessive motion, which could not be resolved in the analysis, were discarded. Distinct and spatially specific clusters of significant functional activity within the primary somatosensory cortices (S1) were identified in 5 study subjects for the left ankle stimulation, in 7 for the right ankle, in 15 for the right wrist, and in 10 for the left wrist. Functional responses to passive wrist movement were located in the hemisphere contralateral to the side of limb stimulation in S1. Although functional responses to ankle movement were similarly located in the contralateral S1 the identified clusters of activity were spatially distinct and located superior and medial to those elicited by wrist movement, as was predicted by a putative homunculus organization (figure 2). In 2 infants, tactile stimulation of the mouth resulted in a bilateral pattern of activation within S1 and extending into the opercular cortex inferior and lateral to those identified following limb stimulation (figure 3).Discussion and Conclusion
We found that functional responses within S1 are somatotopically organized in preterm infants, in keeping with the presence of a homunculus present in the human brain even before the time of normal birth. Whilst it is possible that this may arise in preterm infants due to early exposure to the ex-utero environment, our findings are in keeping with those seen in animals and further support the hypothesis that this specific period represents a critical window for the developing somatosensory system.Acknowledgements
The research is funded by the Engineering and Physical Sciences Council UK (EPSRC) and National Institute of Health Research (NIHR) UK in partnership with King's College London and King's College Hospital NHS Foundation Trust, and in part by the EU - ERC Synergy grant on “Developing Human Connectome Project (dHCP)", as well as EU-FP7 grants PEOPLE-ITN-317488-CONTEST, ICT-601003 BALANCE, ICT-611626 SYMBITRON, and H2020 ICT-644727 COGIMON.References
[1] Seelke et al. PLoS One 2012; 7(2): e32322;
[2] Fox K. J Neurosci 1992; 12(5): 1826-38;
[3] Allievi et al. Ann Biomed Eng 2013; 46(1): 1181-92;
[4] Arichi et al. Neuroimage 2012; 63(1): 663-73;
[5] Allievi et al. Cereb Cortex 2016; 26(1): 402-13.
Figures