2277
Study of functional connectivity of default mode network and frontoparietal network in neuromyelitis optica1Department of Radiology, the First Affiliated Hospital of Chongqing Medical University, Chongqing, People's Republic of China
Synopsis
Neuromyelitis optica (NMO) is an inflammatory, demyelinating syndrome of the central nervous system characterized by severe attacks of optic neuritis and myelitis, but investigators in several studies observed abnormalities in deep gray matter. Functional MRI (fMRI) has the potential to further understanding of the neuropathologic mechanisms of NMO. In the present study, our aim was to investigate patients with NMO-related alterations of brain functional connectivity(FC) in resting state in default mode network (DMN) and frontoparietal network(FPN) using independent component analysis(ICA) and their correlations with clinical features.
Objective
To evaluate the difference of brain default mode network (DMN) and frontoparietal network (FPN) connectivity of neuromyelitis optica (NMO) by using independent component analysis (ICA), and the correlation with clincal score.Methods
Twenty patients with NMO (NMO group) and twenty healthy controls (control group) underwent rest-state fMRI. The fMRI data were analyzed by using GIFT software, and the differences of functional connectivity(FC) between the two groups were compared with SPM8. The correlation between altered parameters of FC and expanded disability status scale (EDSS) scores, disease duration were further explored using the Pearson correlation coefficients.Results
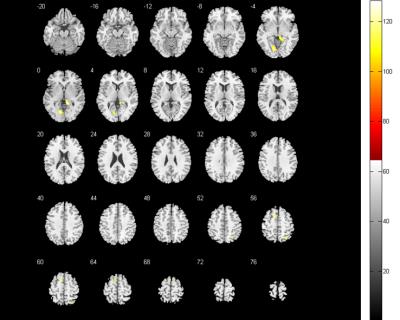
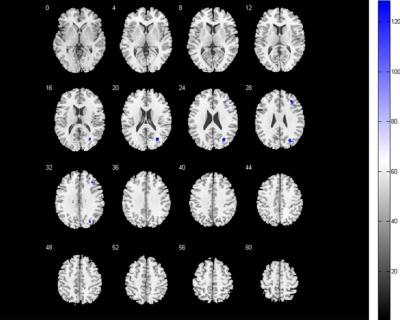
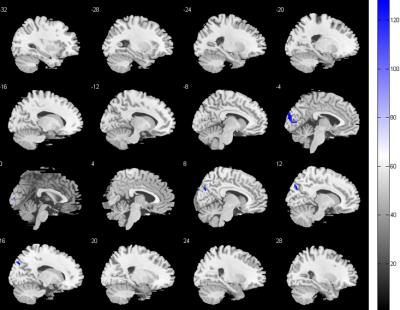
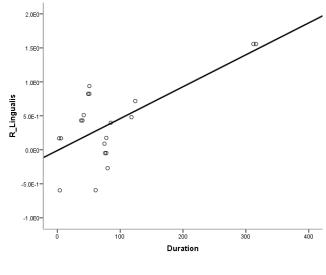
Compared with the control group, the FC score of DMN in NMO group significantly decreased in the right middle frontal cortex and right middle occipital cortex, while increased in bilateral lingual gyrus, extended to right superior parietal lobule and left supplementary motor area; the FC score of FPN decreased in bilateral cuneus cortex, while no FC score increased area. In addition, the FC score changes of right lingual gyrus correlated with disease duration.Conclusion
The FC abnormalities in DMN and FPN in NMO patients may provide a evidence that the spinal cord and optic neuritis not only caused corresponding clincal symptoms but also latent brain damage, valuable method to evaluate neurophysiological changes, which probably caused by a retrograde degeneration of neurons secondary to spinal cord damage or functional network reconstitution, and may help to differential diagnosis with spinal multiple sclerosis.Brain functional networks are complex and interconnected networks with the complexity of damage and compensatory process.Acknowledgements
This study was supported by the National Nature Science Foundation of China under Grant (81371309) and Chongqing Health Bureau Grant (2012-1-017) and the National key clinical specialist construction Programs of China ([2013] 544).The authors would like to thank all the subjects who participated in this study.References
[1] Wingerchuk DM, Hogancamp WE, O'Brien PC, et al. The clinical course of neuromyelitis optica (Devic's syndrome). Neurology,1999,53:1107–1114.
[2] Zhang N, Li YJ, Fu Y, et al. Cognitive impairment in Chinese neuromyelitis optica. Mult Scler, 2015, 21:1839-1846.
[3] Liu Y, Fu Y, Schoonheim MM, et al.Structural MRI substrates of cognitive impairment in neuromyelitis optica. Neurology, 2015, 85:1491-1499.
[4] Rueda Lopes FC, Doting T, Martins C,et al.The role of demyelination in neuromyelitis optica damage:difusion-tensor MR imaging study[J]. Radiology, 2012,263(1):235-242.
[5] Wingerchuk DM, Lennon VA, Pittock SJ, et a1. Revised diagnostic criteria for neuromyelitis optica[J]. Neurology, 2006,66(10):1485-1489.
[6] Yan CG, Zang YF. DPARSF: A MATLAB toolbox for “pipeline”data analysis of resting-state fMRI. Front Syst Neurosci, 2010,4(2):13.
[7] Yah CG, Cheung B, Kelly C, et al. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage, 2013,76(8):183-201.
[8] Beckmann CF, Smith SM. Tensorial extensions of independent component analysis for multisubject FMRI analysis. Neuroimage, 2005,25(1):294-311.
[9] Damoiseaux JS, Rombouts SA, Barkhof F, et al. Consistent resting-state networks across healthy subjects. PNAS, 2006,103(37):13848-13853.
[10] Mantini D, Perrueei MG, Gratta CD, et al. Electrophysiological signatures of resting state networks in the human brain. PNAS, 2007,104(32):13170-13175.
[11] Beckmann CF, Mackay C, Filippin N, et al. Group comparison of resting-state FMRI data using multi-subject ICA and dual regression. Neuroimage, 2009,47(Suppl 1):S148.
[12] Figueroa M, Guo Y, Tselis A, et al. Paraneoplastic neuromyelitis optica spectrum disorder associated with metastaticcarcinoid expressing aquaporin-4. JAMA Neurol, 2014, 71:495-498.
[13] Wingerchuk DM, Lennon VA, Lucchinetti CF, et al. The spectrum of neuromyelitis optica. Lancet Neurol, 2007,6(9):805-815.
[14] DM Wingerchuk,BG Weinshenker. Neuromyelitis optica Clinical predictors of a relapsing course and survival. Neurology, 2003, 60(5):848-853.
[15] Paul F, Jarius S, Aktas O, et al. Antibody to aquaporin 4 in the diagnosis of neuromyelitis optica. PLoS Med, 2007, 4:E133.
[16] McKeown MJ, Makeig S, Brown GG, et al. Analysis of fMRI data by blind separation into independent spatial components. Hum Brain Mapp, 1998,6(3):160-188.
[17] Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease.Ann N Y Acad Sci, 2008,1124(10):1-38.
[18] Y Liu, P Liang, Y Duan, et al. Abnormal baseline brain activity in patients with neuromyelitis optica: A resting-state fMRI study. Eur J of Radiol, 2011, 80(2):407-411.
[19] Menon V, Uddin LQ. Saliency, switching, attention and control: A network model of insula function. Brain Struct Funct, 2010,214(5-6):655-667.
[20] Rocca MA, Agosta F, Mezzapesa DM, et al. A functional MRI study of movement-associated cortical changes in patients with Devic's neuromyelitis optica.Neuroimage, 2004, 21(3):1061-1068.[21] Dobryakova E, Rocca MA,Valsasina P, et al. Abnormalities of the executive control network in multiple sclerosis phenotypes: An fMRI effective connectivity study. Hum Brain Mapp, 2016,37(6):2293-2304.[22] Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci, 2007,27(9):2349-2356.
[23]Tomasi D, Volkow ND. Functional connectivity density mapping. PNAS, 2010,107(21):9885-9890.
Figures