2276
Functionally Informed Fiber Tracking Using Combination of Diffusion and Functional MRI1Department of Computer Science, Chengdu University of Information Technology, Chengdu, People's Republic of China, 2Vanderbilt University Institute of Imaging Science, Nashville, TN, United States, 3Biomedical Engineering, Vanderbilt University, Nashville, TN, United States, 4Radiology and Radiological Sciences, Vanderbilt University, Nashville, TN, United States, 5Electrical Engineering and Computer Science, Vanderbilt University, Nashville, TN, United States
Synopsis
While fiber tractography using diffusion weighted MRI is a primary method that has achieved great success during the past decade, it however suffers from a number of inherent limitations. On the basis of the concept of a spatio-temporal correlation tensor we have introduced previously as a descriptor of the functional architecture in white matter, we propose in this study a novel algorithm for tractography by combing diffusion and functional MRI. Our experimental results show clear improvement of tractography accuracy for fiber tracts in the visual circuit, which demonstrates a great potential for reconstructing functional structure in brain whiter matter.
Purpose
Fiber tractography based on diffusion weighted MRI (DWI) is a primary method for mapping structural connectivity in brain white matter in vivo. In spite of the great success that has been achieved, this method however suffers from a number of inherent limitations. Recently, there has been growing evidence for reliable detection of functional MRI (fMRI) signals in white matter, and we have thus introduced the concept of a spatio-temporal correlation tensor to describe the functional architecture of white matter1. With this concept, we propose a novel algorithm for tractography in which DWI and fMRI are combined using a Bayesian framework. Specifically, the spatio-temporal correlation tensor that characterizes functional architecture is introduced to complement fiber orientation density function (ODF) estimated from DWI data, thus permitting functional information to be incorporated into the fiber tracking process. The proposed method improves anatomical accuracy of tractography for various fiber tracts, and is particularly beneficial to tracking neuronal circuits with distinct functional activities.Method
Full brain MRI data were acquired from ten healthy and right-handed adult volunteers. All imaging was performed on a 3T Philips Achieva scanner (Philips Healthcare, Inc., Best, Netherlands) using a 32-channel head coil. Subjects lay in a supine position with eyes closed except when performing functional tasks. T2*-sensitive data were imaged using a gradient echo (GE), echo planar imaging (EPI) sequence with TR=3 s, TE=45 ms, matrix size=80×80, FOV=240×240 mm2, 34 axial slices of 3 mm thick with zero gap, and 145 volumes. DWI data were obtained using a single-shot, spin echo EPI sequence with b=1000 s/mm2, 32 diffusion-sensitizing directions, TR=8.5 s, TE=65 ms, SENSE factor=3, matrix size=128×128, FOV=256×256, 68 axial slices of 2 mm thick with zero gap. 3D high resolution T1 weighted (T1w) images were acquired using a multi-shot GE sequence at voxel size of 1 mm3.
Each subject was scanned twice for T2*-sensitive imaging with identical parameters, respectively in a resting state and with visual stimulations. The visual stimuli were a flashing (8 Hz) checkerboard, which were presented in a block design format, starting with 30 seconds of blank screen fixation followed by 30 seconds of flashing checkerboard and so on.
All fMRI time series were corrected for slice timing and head motion and smoothed with FWHM=4mm using SPM12. Voxels in each time series were then band-pass filtered to retain frequencies only of 0.01-0.08 Hz which contained the principal stimulus frequency of 0.016 Hz. The filtered images were finally coregistered with the b=0 DWI images, along with the T1w images acquired.
Fiber tracking began with construction of fiber ODF from DWI data based on Q-ball method2. The ODF served as a prior probability of white matter pathways along all possible directions. Meanwhile, spatio-temporal correlation tensors were constructed from T2* images with the method reported in and a Gibbs distribution model was used to model the function information for the maximum likelihood probability3. The posterior distribution was obtained as the product of the likelihood and prior, and was normalized into probability density. The direction of maximum posterior probability was chosen as the optimal functional pathway direction. Fibers were tracked using a probabilistic approach that was modified from Friman et al4.
Result
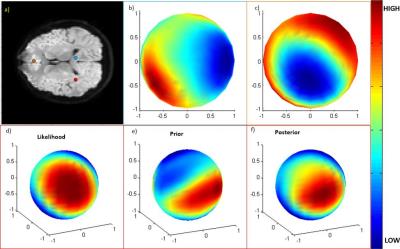
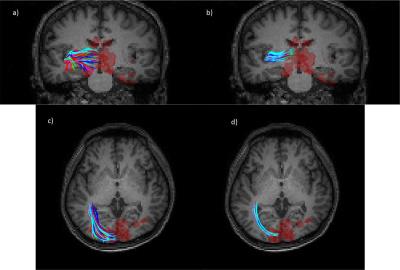
Examples of posterior distribution in some single voxels are shown in Fig. 1. The posterior distribution in the corpus callosum voxel (Fig. 1.b) is concentrated along a specific direction, but is widespread in the gray matter voxel (Fig.1.c). Fig. 1.d-f shows how the posterior is computed in a voxel in the crossing area. Fig.2. show fibers in optic radiations that were tracked from a seed region lying in the course from lateral geniculate nuclei to the visual cortex. The visual cortex was defined as a combination of Brodman areas 17 and 18, and had a correlation coefficient greater than 0.9 with the visual stimuli (red region). As seen, a number of fibers were constructed by DTI tracking only that connected the visual cortex, but some of them meandered away from the main course, indicated by the scattered end points outside the red region. On the other hand, the proposed method yielded clean pathways that connected the visual cortex, with all the end points concentrated in the red region.Conclusion
This work proposed a novel white matter fiber tractography algorithm in which fMRI signals were introduced to improve diffusion MRI fiber tracking. The method proposed holds a great potential for investigating functional structure in brain whiter matter.Acknowledgements
This study is supported by China NSF grants 6162065 and 61602066, and USA NIH grant NS093669 (JCG).References
1. Ding Z et al. Spatio-temporal correlation tensors reveal functional structure in human brain. PLoS One. 2013; 8(12): e82107.
2. Tuch D S. Q-ball imaging. Magnetic resonance in medicine. 2004;52(6): 1358-1372.
3. Poupon et al. Regularization of diffusion-based direction maps for the tracking of brain white matter fascicles. Neuroimage. 2000;12(2): 184-195.
4. Friman O et al. A Bayesian Approach for Stochastic White Matter Tractography. IEEE Transactions on Medical Imaging. 2006; 25(8):965-978
Figures