2275
fMRI of Visual Stimuli in a Tau Model of Alzheimer’s Disease1University College London, London, United Kingdom, 2Eli Lilly and Company, Windlesham, United Kingdom, 3Eli Lilly and Company, Indianapolis, IN, United States
Synopsis
There is a need for non-invasive biomarkers that enable accurate tracking of early, pre-symptomatic phases of Alzheimer’s disease (AD). Previous fMRI studies have differentiated AD patients from healthy controls. However, it is unknown whether the accumulation of tau or amyloid pathology is driving the fMRI irregularity. This is the first study to investigate whether tau pathology alone can modulate task-based BOLD fMRI. We observed stronger BOLD responses to visual stimulation in lateral geniculate nucleus and superior colliculus in the tau cohort, mirroring observations in mild AD and MCI patients.
Purpose
Alzheimer’s disease (AD) is the most common form of dementia, with symptoms that include cognitive impairment and memory loss. There is no definitive diagnostic test for this devastating disease and current AD biomarkers each have their own limitations. For example, CSF sampling for detection of amyloid-β/tau is an invasive procedure which requires a lumbar puncture, while FDG-PET and structural MRI are sensitive to neuronal injury which occurs at a relatively late stage of the disease. The development of a safe and non-invasive early biomarker may provide an early window for therapeutic intervention in order to delay or prevent clinical symptoms occurring later in life.
fMRI has been investigated as a possible biomarker of AD. The results are promising, with differences in fMRI responses observed between AD patients and healthy controls.1 However, how the major molecular correlates of AD (amyloid-β/tau) may act to modulate the fMRI signal is poorly understood. In order to better understand the interaction of pathology and the fMRI signal, we characterized the fMRI signature to visual stimulation in a mouse model of tauopathy, the rTg4510,2 enabling the novel assessment of the relationship between tau pathology alone and clinically relevant non-invasive fMRI measures.
Methods
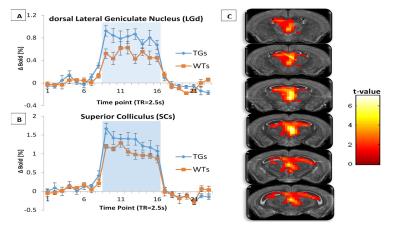
Task-based fMRI using visual stimuli were acquired at 9.4T from 10 litter-matched healthy wild-type (WT) and 8 rTg4510 mice at 7.5 months of age. Anaesthesia was induced with 2% isoflurane and maintained with medetomidine [0.4 mg/kg bolus, 0.8 mg/kg/hr infusion initiated 10 min after bolus] and isoflurane [0.5% in a mixture of 0.8L/min medical air and 0.2L/min O2]. Animals were presented with a diffuse flashing white light at 2Hz for 20s followed by 40s resting period. fMRI data were acquired using an interleaved snapshot GE-EPI sequence.3 Region of interest (ROI) analysis was performed using MarsBar toolbox on brain regions important in the visual processing pathway.3 BOLD timecourses were extracted from structural ROIs automatically registered to and derived from the Allen mouse brain atlas.4Results
In order to directly compare differences in the BOLD response to visual stimuli between the two groups, group level mixed effects analysis was performed using SPM. A greater ‘activation’ in the midbrain region (LGd, SCs) was observed in the rTg4510 group relative to the WT group (fig1-C). From the mean BOLD timecourses (fig1-A/B), it can be seen that the transgenic group overall demonstrate a stronger response to visual stimulation compared to WTs in the midbrain (56% and 27% greater in LGd and SCs respectively).Discussion
Our data provides evidence that the rTg4510 mice demonstrate a stronger midbrain BOLD response as a result of visual stimulation. This may be a compensatory mechanism due to the underlying pathology. Accordingly, a compensatory mechanism associated with semantic memory impairment has been previously proposed where patients with mild AD demonstrated increased BOLD responses in language-related frontal brain regions compared to healthy control participants.1 Interestingly, one study investigating an amyloid model of AD with in-vivo optical imaging techniques has demonstrated an augmented hemodynamic response to electrical stimulation of the hind-paw.5Conclusion
This study has successfully characterized differences in fMRI signals due to tauopathy (rTg4510) in major components of the visual processing pathway (SCs/LGd); implicating buildup of tau in the derangement of the visual system. This could be a stepping stone for progressing the utility of fMRI as a valuable non-invasive biomarker for the diagnosis and tracking of AD.Acknowledgements
This work is supported by the EPSRC-funded UCL Centre for Doctoral Training in Medical Imaging (EP/L016478/1) and the Department of Health’s NIHR-funded Biomedical Research Centre at University College London Hospitals.References
[1]- Wierenga, C.E., Stricker, N.H., McCauley, A., Simmons, A., Jak, A.J., Chang, Y.L., Nation, D.A., Bangen, K.J., Salmon, D.P., Bondi, M.W., 2011. Altered brain response for semantic knowledge in Alzheimer's disease. Neuropsychologia 49:392–404.
[2]- Wells, J.A., O'Callaghan, J.M., Holmes, H.E., Powell, N.M., Johnson, R.A., Siow, B., Torrealdea, F., Ismail, O., Walker-Samuel, S., Golay, X., Rega, M., Richardson, S., Modat, M., Cardoso, M.J., Ourselin, S., Schwarz, A.J., Ahmed, Z., Murray, T.K., O'Neill, M.J., Collins, E.C., Colgan, N., Lythgoe, M.F., 2015. In vivo imaging of tau pathology using multi-parametric quantitative MRI. NeuroImage. 111 pp. 369–378
[3]- Niranjan, A., Christie, I.N., Solomon, S.G., Wells, J.A., Lythgoe, M.F., 2016. fMRI mapping of the visual system in the mouse brain with interleaved snapshot GE-EPI. Nueroimage. 139 pp. 337-345
[4]- Lein, E.S., Hawrylycz, M.J., Ao, N., Ayres, M., Bensinger, A., Bernard, A., Boe, A.F., Boguski, M.S., Brockway, K.S., Byrnes, E.J., Chen, L., Chen, L., Chen, T.-M., Chi Chin, M., Chong, J., Crook, B.E., Czaplinska, A., Dang, C.N., Datta, S., Dee, N.R., Desaki, A.L., Desta, T., Diep, E., Dolbeare, T.A., Donelan, M.J., Dong, H.-W., Dougherty, J.G., Duncan, B.J., Ebbert, A.J., Eichele, G., Estin, L.K., Faber, C., Facer, B.A., Fields, R., Fischer, S.R., Fliss, T.P., Frensley, C., Gates, S.N., Glattfelder, K.J., Halverson, K.R., Hart, M.R., Hohmann, J.G., Howell, M.P., Jeung, D.P., Johnson, R.A., Karr, P.T., Kawal, R., Kidney, J.M., Knapik, R.H., Kuan, C.L., Lake, J.H., Laramee, A.R., Larsen, K.D., Lau, C., Lemon, T.A., Liang, A.J., Liu, Y., Luong, L.T., Michaels, J., Morgan, J.J., Morgan, R.J., Mortrud, M.T., Mosqueda, N.F., Ng, L.L., Ng, R., Orta, G.J., Overly, C.C., Pak, T.H., Parry, S.E., Pathak, S.D., Pearson, O.C., Puchalski, R.B., Riley, Z.L., Rockett, H.R., Rowland, S.A., Royall, J.J., Ruiz, M.J., Sarno, N.R., Schaffnit, K., Shapovalova, N.V., Sivisay, T., Slaughterbeck, C.R., Smith, S.C., Smith, K.A., Smith, B.I., Sodt, A.J., Stewart, N.N., Stumpf, K.-R., Sunkin, S.M., Sutram, M., Tam, A., Teemer, C.D., Thaller, C., Thompson, C.L., Varnam, L.R., Visel, A., Whitlock, R.M., Wohnoutka, P.E., Wolkey, C.K., Wong, V.Y., Wood, M., Yaylaoglu, M.B., Young, R.C., Youngstrom, B.L., Feng Yuan, X., Zhang, B., Zwingman, T.A., Jones, A.R.,2007. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 445:168–176.
[5]- Kim, J., Jeong, Y., 2013. Augmentation of sensory-evoked hemodynamic response in an early Alzheimer's disease mouse model. J Alzheimers Dis. 37(4):857-68.
Figures